Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis
- PMID: 30661024
- PMCID: PMC7642975
- DOI: 10.1136/thoraxjnl-2018-211845
Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis
Abstract
Background: Mechanisms that facilitate early infection and inflammation in cystic fibrosis (CF) are unclear. We previously demonstrated that children with CF and parental-reported secondhand smoke exposure (SHSe) have increased susceptibility to bacterial infections. SHSe hinders arachidonic acid (AA) metabolites that mediate immune function in patients without CF, and may influence CF immune dysfunction. We aimed to define SHSe's impact on inflammation mediators and infection in children with CF.
Methods: Seventy-seven children with CF <10 years of age (35 infants <1 year; 42 children 1-10 years) were enrolled and hair nicotine concentrations measured as an objective surrogate of SHSe. AA signalling by serum and macrophage lipidomics, inflammation using blood transcriptional profiles and in vitro macrophage responses to bacterial infection after SHSe were assessed.
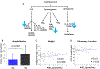
Results: Hair nicotine concentrations were elevated in 63% of patients. Of the AA metabolites measured by plasma lipidomics, prostaglandin D2 (PGD2) concentrations were decreased in children with CF exposed to SHSe, and associated with more frequent hospitalisations (p=0.007) and worsened weight z scores (p=0.008). Children with CF exposed to SHSe demonstrated decreased expression of the prostaglandin genes PTGES3 and PTGR2 and overexpression of inflammatory pathways. These findings were confirmed using an in vitro model, where SHSe was associated with a dose-dependent decrease in PGD2 and increased methicillin-resistant Staphylococcus aureus survival in human CF macrophages.
Conclusions: Infants and young children with CF and SHSe have altered AA metabolism and dysregulated inflammatory gene expression resulting in impaired bacterial clearance. Our findings identified potential therapeutic targets to halt early disease progression associated with SHSe in the young population with CF.
Keywords: cystic fibrosis; macrophage biology; paediatric lung disaese; tobacco and the lung.
© Author(s) (or their employer(s)) 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





Comment in
-
Impact of secondhand smoke on cystic fibrosis: is there a link to fatty acid metabolism?Thorax. 2019 Jun;74(6):529-530. doi: 10.1136/thoraxjnl-2019-213042. Epub 2019 May 2. Thorax. 2019. PMID: 31048510 No abstract available.
Similar articles
-
Metabolomics profiling of tobacco exposure in children with cystic fibrosis.J Cyst Fibros. 2020 Sep;19(5):791-800. doi: 10.1016/j.jcf.2020.05.003. Epub 2020 May 30. J Cyst Fibros. 2020. PMID: 32487493 Free PMC article.
-
Lung function and secondhand smoke exposure among children with cystic fibrosis: A Bayesian meta-analysis.J Cyst Fibros. 2023 Jul;22(4):694-701. doi: 10.1016/j.jcf.2023.04.020. Epub 2023 May 2. J Cyst Fibros. 2023. PMID: 37142525 Free PMC article.
-
Age and environmental exposures influence the fecal bacteriome of young children with cystic fibrosis.Pediatr Pulmonol. 2020 Jul;55(7):1661-1670. doi: 10.1002/ppul.24766. Epub 2020 Apr 10. Pediatr Pulmonol. 2020. PMID: 32275127 Free PMC article.
-
The Impact of Secondhand Smoke Exposure on Children with Cystic Fibrosis: A Review.Int J Environ Res Public Health. 2016 Oct 12;13(10):1003. doi: 10.3390/ijerph13101003. Int J Environ Res Public Health. 2016. PMID: 27754353 Free PMC article. Review.
-
Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease.JAMA. 2008 Jan 30;299(4):417-24. doi: 10.1001/jama.299.4.417. JAMA. 2008. PMID: 18230779 Free PMC article.
Cited by
-
Metabolomics profiling of tobacco exposure in children with cystic fibrosis.J Cyst Fibros. 2020 Sep;19(5):791-800. doi: 10.1016/j.jcf.2020.05.003. Epub 2020 May 30. J Cyst Fibros. 2020. PMID: 32487493 Free PMC article.
-
Secondhand vape exposure regulation of CFTR and immune function in cystic fibrosis.Am J Physiol Lung Cell Mol Physiol. 2025 Mar 1;328(3):L324-L333. doi: 10.1152/ajplung.00328.2024. Epub 2025 Jan 21. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39836014 Free PMC article.
-
Lung function and secondhand smoke exposure among children with cystic fibrosis: A Bayesian meta-analysis.J Cyst Fibros. 2023 Jul;22(4):694-701. doi: 10.1016/j.jcf.2023.04.020. Epub 2023 May 2. J Cyst Fibros. 2023. PMID: 37142525 Free PMC article.
-
Identification of key metabolism-related genes and pathways in spontaneous preterm birth: combining bioinformatic analysis and machine learning.Front Endocrinol (Lausanne). 2024 Aug 20;15:1440436. doi: 10.3389/fendo.2024.1440436. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39229380 Free PMC article.
-
Aiming to Improve Equity in Pulmonary Health: Cystic Fibrosis.Clin Chest Med. 2023 Sep;44(3):555-573. doi: 10.1016/j.ccm.2023.03.011. Epub 2023 May 8. Clin Chest Med. 2023. PMID: 37517835 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
