Discovery of Distinct Immune Phenotypes Using Machine Learning in Pulmonary Arterial Hypertension
- PMID: 30661465
- PMCID: PMC6428071
- DOI: 10.1161/CIRCRESAHA.118.313911
Discovery of Distinct Immune Phenotypes Using Machine Learning in Pulmonary Arterial Hypertension
Abstract
Rationale: Accumulating evidence implicates inflammation in pulmonary arterial hypertension (PAH) and therapies targeting immunity are under investigation, although it remains unknown if distinct immune phenotypes exist.
Objective: Identify PAH immune phenotypes based on unsupervised analysis of blood proteomic profiles.
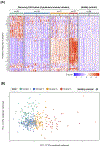
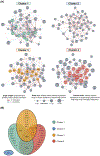
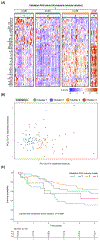
Methods and results: In a prospective observational study of group 1 PAH patients evaluated at Stanford University (discovery cohort; n=281) and University of Sheffield (validation cohort; n=104) between 2008 and 2014, we measured a circulating proteomic panel of 48 cytokines, chemokines, and factors using multiplex immunoassay. Unsupervised machine learning (consensus clustering) was applied in both cohorts independently to classify patients into proteomic immune clusters, without guidance from clinical features. To identify central proteins in each cluster, we performed partial correlation network analysis. Clinical characteristics and outcomes were subsequently compared across clusters. Four PAH clusters with distinct proteomic immune profiles were identified in the discovery cohort. Cluster 2 (n=109) had low cytokine levels similar to controls. Other clusters had unique sets of upregulated proteins central to immune networks-cluster 1 (n=58; TRAIL [tumor necrosis factor-related apoptosis-inducing ligand], CCL5 [C-C motif chemokine ligand 5], CCL7, CCL4, MIF [macrophage migration inhibitory factor]), cluster 3 (n=77; IL [interleukin]-12, IL-17, IL-10, IL-7, VEGF [vascular endothelial growth factor]), and cluster 4 (n=37; IL-8, IL-4, PDGF-β [platelet-derived growth factor beta], IL-6, CCL11). Demographics, PAH clinical subtypes, comorbidities, and medications were similar across clusters. Noninvasive and hemodynamic surrogates of clinical risk identified cluster 1 as high-risk and cluster 3 as low-risk groups. Five-year transplant-free survival rates were unfavorable for cluster 1 (47.6%; 95% CI, 35.4%-64.1%) and favorable for cluster 3 (82.4%; 95% CI, 72.0%-94.3%; across-cluster P<0.001). Findings were replicated in the validation cohort, where machine learning classified 4 immune clusters with comparable proteomic, clinical, and prognostic features.
Conclusions: Blood cytokine profiles distinguish PAH immune phenotypes with differing clinical risk that are independent of World Health Organization group 1 subtypes. These phenotypes could inform mechanistic studies of disease pathobiology and provide a framework to examine patient responses to emerging therapies targeting immunity.
Keywords: classification; cytokine; inflammation; interleukin; phenotype; pulmonary hypertension.
Figures



Comment in
-
Precision Medicine in Pulmonary Arterial Hypertension.Circ Res. 2019 Mar 15;124(6):832-833. doi: 10.1161/CIRCRESAHA.119.314757. Circ Res. 2019. PMID: 30870130 Free PMC article. No abstract available.
References
-
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM and Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

