Electroacupuncture Facilitates the Integration of Neural Stem Cell-Derived Neural Network with Transected Rat Spinal Cord
- PMID: 30661994
- PMCID: PMC6373172
- DOI: 10.1016/j.stemcr.2018.12.015
Electroacupuncture Facilitates the Integration of Neural Stem Cell-Derived Neural Network with Transected Rat Spinal Cord
Abstract
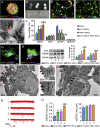
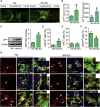
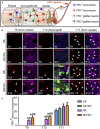
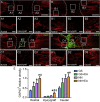
The hostile environment of an injured spinal cord makes it challenging to achieve higher viability in a grafted tissue-engineered neural network used to reconstruct the spinal cord circuit. Here, we investigate whether cell survival and synaptic transmission within an NT-3 and TRKC gene-overexpressing neural stem cell-derived neural network scaffold (NN) transplanted into transected spinal cord could be promoted by electroacupuncture (EA) through improving the microenvironment. Our results showed that EA facilitated the cell survival, neuronal differentiation, and synapse formation of a transplanted NN. Pseudorabies virus tracing demonstrated that EA strengthened synaptic integration of the transplanted NN with the host neural circuit. The combination therapy also promoted axonal regeneration, spinal conductivity, and functional recovery. The findings highlight EA as a potential and safe supplementary therapeutic strategy to reinforce the survival and synaptogenesis of a transplanted NN as a neuronal relay to bridge the two severed ends of an injured spinal cord.
Keywords: electroacupuncture; neural stem cells; neuronal relay; neurotrophin-3; spinal cord injury; tissue-engineered neural network.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Agbay A., Edgar J.M., Robinson M., Styan T., Wilson K., Schroll J., Ko J., Khadem Mohtaram N., Jun M.B., Willerth S.M. Biomaterial strategies for delivering stem cells as a treatment for spinal cord injury. Cells Tissues Organs. 2016;202:42–51. - PubMed
-
- Bareyre F.M., Kerschensteiner M., Raineteau O., Mettenleiter T.C., Weinmann O., Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. - PubMed
-
- Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

