Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets
- PMID: 30662308
- PMCID: PMC6323142
- DOI: 10.1016/j.jsps.2018.07.020
Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets
Abstract
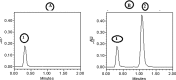
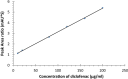
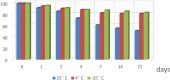
A new selective and sensitive high-performance liquid chromatography (HPLC) method was developed for the quantification of diclofenac sodium (DS) in pharmaceutical dosage form using lidocaine as internal standard (IS). Chromatographic separation was achieved on a symmetry C18 column (4.6 mm × 150 mm, 3 μm spherical particles) using 0.05 M orthophosporic (pH 2.0) 35% and acetonitrile as 65%, as the mobile phase at a flow rate of 2.0 mL/min and monitored at 210 nm. The run time was 2 min. The method was validated to fulfill International Conference on Harmonisation (ICH) requirements and this validation included specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision and robustness. The calibration curve was linear over the concentration range from 10 to 200 µg/ml, and lower limit of detection of 12.5 ng/ml. The accuracy and precision of the method were within the acceptable limit of ±20% at the lower limit of quantitation and ±15% at other concentrations. Diclofenac was unstable at room temperature it showed more than 25% loss after 24 h. While, DS is very stable at refrigerator 4 °C auto-sampler, freeze/thaw cycles and 30 days storage in a freezer at -35 ± 2 °C. All results were acceptable and this confirmed that the method is suitable for its intended use in routine quality control and assay of drugs.
Keywords: Diclofenac; HPLC; Reverse phase high performance liquid chromatography.
Figures
Similar articles
-
Rapid Development and Validation of Improved Reversed-Phase High-performance Liquid Chromatography Method for the Quantification of Mangiferin, a Polyphenol Xanthone Glycoside in Mangifera indica.Pharmacognosy Res. 2017 Apr-Jun;9(2):215-219. doi: 10.4103/0974-8490.204652. Pharmacognosy Res. 2017. PMID: 28539748 Free PMC article.
-
Development And Validation Of Rp-Hplc Method For The Estimation Of Tenofovir And Emtricitabine In Bulk And Pharmaceutical Dosage Form.Curr Drug Res Rev. 2023 Jun 2. doi: 10.2174/2589977515666230602151222. Online ahead of print. Curr Drug Res Rev. 2023. PMID: 37278041
-
Validation of the HPLC Analytical Method for the Determination of Chemical and Radiochemical Purity of Ga-68-DOTATATE.Indian J Nucl Med. 2023 Jan-Mar;38(1):1-7. doi: 10.4103/ijnm.ijnm_11_22. Epub 2023 Feb 24. Indian J Nucl Med. 2023. PMID: 37180199 Free PMC article.
-
Characterization and Analytical Method Validation for Potential Impurities of a Merchantability Drug Substance Fluoxetine HCl.Biomed Chromatogr. 2025 Feb;39(2):e6069. doi: 10.1002/bmc.6069. Biomed Chromatogr. 2025. PMID: 39748240 Free PMC article.
-
Method Development and Validation for the Determination of Caffeine: An Alkaloid from Coffea arabica by High-performance Liquid Chromatography Method.Pharmacognosy Res. 2018 Jan-Mar;10(1):88-91. doi: 10.4103/pr.pr_79_17. Pharmacognosy Res. 2018. PMID: 29568193 Free PMC article.
Cited by
-
Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles.Saudi Pharm J. 2019 Jan;27(1):82-87. doi: 10.1016/j.jsps.2018.08.001. Epub 2018 Aug 29. Saudi Pharm J. 2019. PMID: 30662310 Free PMC article.
-
Nanomaterials as catalysts for the sensitive and selective determination of diclofenac.ADMET DMPK. 2023 Nov 16;12(1):151-165. doi: 10.5599/admet.2116. eCollection 2024. ADMET DMPK. 2023. PMID: 38560716 Free PMC article. Review.
-
Application of Thin-Layer Chromatography in Combination with Densitometry for the Determination of Diclofenac in Enteric Coated Tablets.Pharmaceuticals (Basel). 2019 Dec 16;12(4):183. doi: 10.3390/ph12040183. Pharmaceuticals (Basel). 2019. PMID: 31888153 Free PMC article.
-
A Validated RP-HPLC Method for Simultaneous Determination of Cefixime and Clavulanic Acid Powder in Pediatric Oral Suspension.Int J Anal Chem. 2022 Jul 1;2022:8331762. doi: 10.1155/2022/8331762. eCollection 2022. Int J Anal Chem. 2022. PMID: 35814262 Free PMC article.
-
Highly sensitive and selective colorimetric detection of dual metal ions (Hg2+ and Sn2+) in water: an eco-friendly approach.RSC Adv. 2021 Apr 20;11(24):14700-14709. doi: 10.1039/d0ra09926k. eCollection 2021 Apr 15. RSC Adv. 2021. PMID: 35424016 Free PMC article.
References
-
- Aldwaikat M., Alarjah M. Investigating the sonophoresis effect on the permeation of diclofenac sodium using 3D skin equivalent. Ultrason. Sonochem. 2015;22:580–587. - PubMed
-
- Basusarkar A. Stability studies on diclofenac cream. Int. J. Adv. Pharm. Anal. 2011;1:1–3.
-
- Bhattacharya S.S., Banerjee S., Ghosh A.K., Chattopadhyay P., Verma A., Ghosh A. A RP-HPLC method for quantification of diclofenac sodium released from biological macromolecules. Int. J. Biol. Macromol. 2013;58:354–359. - PubMed
-
- Chaudhary H., Kohli K., Amin S., Rathee P., Kumar V. Optimization and formulation design of gels of Diclofenac and Curcumin for transdermal drug delivery by Box-Behnken statistical design. J. Pharm. Sci. 2011;100:580–593. - PubMed
LinkOut - more resources
Full Text Sources

