The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection
- PMID: 30662442
- PMCID: PMC6328441
- DOI: 10.3389/fimmu.2018.03083
The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection
Abstract
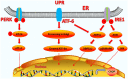
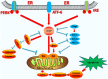
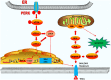
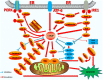
Apoptosis is a form of cell death by which the body maintains the homeostasis of the internal environment. Apoptosis is an initiative cell death process that is controlled by genes and is mainly divided into endogenous pathways (mitochondrial pathway), exogenous pathways (death receptor pathway), and apoptotic pathways induced by endoplasmic reticulum (ER) stress. The homeostasis imbalance in ER results in ER stress. Under specific conditions, ER stress can be beneficial to the body; however, if ER protein homeostasis is not restored, the prolonged activation of the unfolded protein response may initiate apoptotic cell death via the up-regulation of the C/EBP homologous protein (CHOP). CHOP plays an important role in ER stress-induced apoptosis and this review focuses on its multifunctional roles in that process, as well as its role in apoptosis during microbial infection. We summarize the upstream and downstream pathways of CHOP in ER stress induced apoptosis. We also focus on the newest discoveries in the functions of CHOP-induced apoptosis during microbial infection, including DNA and RNA viruses and some species of bacteria. Understanding how CHOP functions during microbial infection will assist with the development of antimicrobial therapies.
Keywords: C/EBP homologous protein; apoptosis; bacteria; endoplasmic reticulum stress; microorganisms; virus.
Figures
References
-
- Luethy JD, Holbrook NJ. Activation of the gadd153 promoter by genotoxic agents: a rapid and specific response to DNA damage. Cancer Res. (1992) 52:5–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

