Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy
- PMID: 30662558
- PMCID: PMC6332787
- DOI: 10.7150/thno.29431
Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy
Abstract
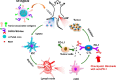
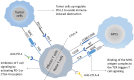
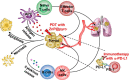
Owing to the fast-paced growth and cross-infiltration of oncology, immunology and molecular biology, tumor immunotherapy technology represented by immune checkpoint blockade and chimeric antigen receptor (CAR) T cell therapy has lately made remarkable advancements. In comparison with traditional chemotherapy, immunotherapy has the potential to elicit a stronger sustained antitumor immune response in those patients who have advanced malignant malignancies. In spite of the advancements made, a significant number of clinical research works have validated that an extensive proportion of cancer patients still manifest insensitivity to immunotherapy, primarily because of the immunomodulatory interactions between tumor cells and the immunosuppressive tumor microenvironment (TME), together mediating the immune tolerance of tumors and accordingly impacting the positive response to immunotherapy. The intricate immunosuppressive networks formed by stromal cells, inflammatory cells, vasculature, extracellular matrix (ECM), and their secreted cytokines in the TME, play a pivotal role in tumor immune escape. Specific blocking of inhibition pathways in the TME is expected to effectively prevent immune escape and tolerance of tumor cells in addition to their metastasis, accordingly improving the antitumor immune response at various phases of tumor growth. Emerging nanoscale targeted drug carriers truly suit this specific requirement due to their specificity, biocompatibility, and convenience of production. This review emphasizes recent attempts to remodel the tumor immune microenvironment using novel nanoparticles, which include specifically eliminating immunosuppressive cells, reprogramming immune regulatory cells, promoting inflammatory cytokines and blocking immune checkpoints. Targeted remodeling of the immunosuppressive TME using well-designed and fabricated nanoparticles provides a promising strategy for improving the effectiveness of current immunotherapy and is greatly significant.
Keywords: cancer; immunotherapy; nanoparticles; tumor microenvironment; vaccines.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

