Snap-jaw morphology is specialized for high-speed power amplification in the Dracula ant, Mystrium camillae
- PMID: 30662749
- PMCID: PMC6304126
- DOI: 10.1098/rsos.181447
Snap-jaw morphology is specialized for high-speed power amplification in the Dracula ant, Mystrium camillae
Abstract
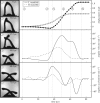
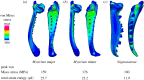
What is the limit of animal speed and what mechanisms produce the fastest movements? More than natural history trivia, the answer provides key insight into the form-function relationship of musculoskeletal movement and can determine the outcome of predator-prey interactions. The fastest known animal movements belong to arthropods, including trap-jaw ants, mantis shrimp and froghoppers, that have incorporated latches and springs into their appendage systems to overcome the limits of muscle power. In contrast to these examples of power amplification, where separate structures act as latch and spring to accelerate an appendage, some animals use a 'snap-jaw' mechanism that incorporates the latch and spring on the accelerating appendage itself. We examined the kinematics and functional morphology of the Dracula ant, Mystrium camillae, who use a snap-jaw mechanism to quickly slide their mandibles across each other similar to a finger snap. Kinematic analysis of high-speed video revealed that snap-jaw ant mandibles complete their strike in as little as 23 µsec and reach peak velocities of 90 m s-1, making them the fastest known animal appendage. Finite-element analysis demonstrated that snap-jaw mandibles were less stiff than biting non-power-amplified mandibles, consistent with their use as a flexible spring. These results extend our understanding of animal speed and demonstrate how small changes in morphology can result in dramatic differences in performance.
Keywords: ants; finite-element analysis; functional morphology; microCT; power amplification.
Conflict of interest statement
We declare we have no competing interests.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
