Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals
- PMID: 30662955
- PMCID: PMC6333230
- DOI: 10.1021/acsenergylett.8b01669
Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals
Abstract
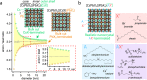
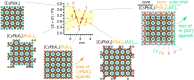
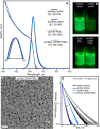
Colloidal lead halide perovskite nanocrystals (NCs) have recently emerged as versatile photonic sources. Their processing and luminescent properties are challenged by the lability of their surfaces, i.e., the interface of the NC core and the ligand shell. On the example of CsPbBr3 NCs, we model the nanocrystal surface structure and its effect on the emergence of trap states using density functional theory. We rationalize the typical observation of a degraded luminescence upon aging or the luminescence recovery upon postsynthesis surface treatments. The conclusions are corroborated by the elemental analysis. We then propose a strategy for healing the surface trap states and for improving the colloidal stability by the combined treatment with didodecyldimethylammonium bromide and lead bromide and validate this approach experimentally. This simple procedure results in robust colloids, which are highly pure and exhibit high photoluminescence quantum yields of up to 95-98%, retained even after three to four rounds of washing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15 (6), 3692–3696. 10.1021/nl5048779. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
