APT-weighted MRI: Techniques, current neuro applications, and challenging issues
- PMID: 30663162
- PMCID: PMC6625919
- DOI: 10.1002/jmri.26645
APT-weighted MRI: Techniques, current neuro applications, and challenging issues
Abstract
Amide proton transfer-weighted (APTw) imaging is a molecular MRI technique that generates image contrast based predominantly on the amide protons in mobile cellular proteins and peptides that are endogenous in tissue. This technique, the most studied type of chemical exchange saturation transfer imaging, has been used successfully for imaging of protein content and pH, the latter being possible due to the strong dependence of the amide proton exchange rate on pH. In this article we briefly review the basic principles and recent technical advances of APTw imaging, which is showing promise clinically, especially for characterizing brain tumors and distinguishing recurrent tumor from treatment effects. Early applications of this approach to stroke, Alzheimer's disease, Parkinson's disease, multiple sclerosis, and traumatic brain injury are also illustrated. Finally, we outline the technical challenges for clinical APT-based imaging and discuss several controversies regarding the origin of APTw imaging signals in vivo. Level of Evidence: 3 Technical Efficacy Stage: 3 J. Magn. Reson. Imaging 2019;50:347-364.
Keywords: APT-weighted imaging; CEST imaging; brain tumor; molecular imaging; stroke.
© 2019 International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
J.Z. and P.C.M.v.Z. are co-inventors on a patent for the APT MRI technology. This patent is owned and managed by Johns Hopkins University. P.C.M.v.Z. is a paid lecturer for Philips Medical Systems. This arrangement has been approved by The Johns Hopkins University in accordance with its Conflict of Interest policies.
Figures



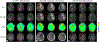
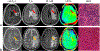
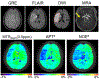
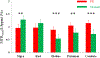


References
-
- Forsen S, Hoffman RA. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 1963;39:2892–901.
-
- Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143:79–87. - PubMed
-
- Zhou J, van Zijl PC. Chemical exchange saturation transfer imaging and spectroscopy. Progr NMR Spectr 2006;48:109–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

