Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors
- PMID: 30663185
- PMCID: PMC6800243
- DOI: 10.1002/anie.201814098
Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors
Abstract
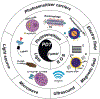
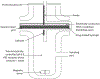
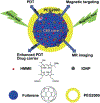
The viable use of photodynamic therapy (PDT) in cancer therapy has never been fully realized because of its undesirable effects on healthy tissues. Herein we summarize some physicochemical factors that can make PDT a more viable and effective option to provide future oncological patients with better-quality treatment options. These physicochemical factors include light sources, photosensitizer (PS) carriers, microwaves, electric fields, magnetic fields, and ultrasound. This Review is meant to provide current information pertaining to PDT use, including a discussion of in vitro and in vivo studies. Emphasis is placed on the physicochemical factors and their potential benefits in overcoming the difficulty in transitioning PDT into the medical field. Many advanced techniques, such as employing X-rays as a light source, using nanoparticle-loaded stem cells and bacteriophage bio-nanowires as a photosensitizer carrier, as well as integration with immunotherapy, are among the future directions.
Keywords: cancer therapy; medical chemistry; microwaves; photodynamic therapy (PDT); ultrasound.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures








References
-
- Qiu P, Yang M, Qu X, Huai Y, Zhu Y, Mao C, Biomaterials 2016, 104, 138–144; - PMC - PubMed
- Wan SS, Zeng JY, Cheng H, Zhang XZ, Biomaterials 2018, 185, 51–62; - PubMed
- Zhang D, Feng F, Li QL, Wang XY, Yao L, Biomaterials 2018, 173, 22–33; - PubMed
- Shaughnessy MJO, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, Monette S, Kim K, Corradi RB, Scherz A, Coleman JA, Clin. Cancer Res 2018, 24, 592–599; - PMC - PubMed
- Ryu TK, Baek SW, Kang RH, Jeong KY, Jun DR, Choi SW, J. Controlled Release 2018, 270, 237–245; - PubMed
- Baran TM, Lasers Surg. Med 2018, 50, 476–482; - PubMed
- Sun XH, Zebibula A, Dong XB, Li GH, Zhang GX, Zhang DQ, Qian J, He SL, Nano Res 2018, 11, 2756–2770.
-
- Ngweniform P, Abbineni G, Cao B, Mao C, Small 2009, 5, 1963–1969; - PubMed
- Ngweniform P, Li D, Mao C, Soft Matter 2009, 5, 954–956.
-
- Xia L, Kong X, Liu X, Tu L, Zhang Y, Chang Y, Liu K, Shen D, Zhao H, Zhang H, Biomaterials 2014, 35, 4146–4156; - PubMed
- Staicu A, Pascu A, Nuta A, Sorescu A, Raditoiu V, Pascu ML, Rom. Rep. Phys 2013, 65, 1032–1051.
-
- Krummenauer F, Braun M, Dick HB, Eur. J. Ophthalmol 2005, 15, 74–80. - PubMed
-
- Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X, Biomaterials 2016, 97, 62–73; - PMC - PubMed
- Master A, Livingston M, Gupta AS, J. Controlled Release 2013, 168, 88–102; - PMC - PubMed
- Khurshid A, Firdous S, Ahmat L, Ferraria J, Vollet-Filho JD, Kurachi C, Bagneto VS, Nawaz M, Ikram M, Ahmad M, Laser Phys 2012, 22, 317–321;
- Succo G, Rosso S, Fadda GL, Fantini M, Crosetti E, Photodiagn. Photodyn. Ther 2014, 11, 63–70; - PubMed
- Cao B, Yang M, Zhu Y, Qu X, Mao C, Adv. Mater 2014, 26, 4627–4631; - PMC - PubMed
- Sreeram KJ, Narayan S, Abbineni G, Hayhurst A, Mao C, Mol. Cancer Ther 2010, 9, 2524–2535. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

