Humanized NOD/SCID/IL2rγnull (hu-NSG) Mouse Model for HIV Replication and Latency Studies
- PMID: 30663638
- PMCID: PMC6559246
- DOI: 10.3791/58255
Humanized NOD/SCID/IL2rγnull (hu-NSG) Mouse Model for HIV Replication and Latency Studies
Abstract
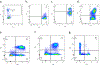
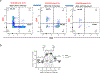
Ethical regulations and technical challenges for research in human pathology, immunology, and therapeutic development have placed small animal models in high demand. With a close genetic and behavioral resemblance to humans, small animals such as the mouse are good candidates for human disease models, through which human-like symptoms and responses can be recapitulated. Further, the mouse genetic background can be altered to accommodate diverse demands. The NOD/SCID/IL2rγnull (NSG) mouse is one of the most widely used immunocompromised mouse strains; it allows engraftment with human hematopoietic stem cells and/or human tissues and the subsequent development of a functional human immune system. This is a critical milestone in understanding the prognosis and pathophysiology of human-specific diseases such as HIV/AIDS and aiding the search for a cure. Herein, we report a detailed protocol for generating a humanized NSG mouse model (hu-NSG) by hematopoietic stem cell transplantation into a radiation-conditioned neonatal NSG mouse. The hu-NSG mouse model shows multi-lineage development of transplanted human stem cells and susceptibility to HIV-1 viral infection. It also recapitulates key biological characteristics in response to combinatorial antiretroviral therapy (cART).
Conflict of interest statement
Disclosures
The authors disclose no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
