Imaging the Injured Lung: Mechanisms of Action and Clinical Use
- PMID: 30664057
- PMCID: PMC6692186
- DOI: 10.1097/ALN.0000000000002583
Imaging the Injured Lung: Mechanisms of Action and Clinical Use
Erratum in
-
Imaging the Injured Lung: Mechanisms of Action and Clinical Use: Erratum.Anesthesiology. 2019 Aug;131(2):451. doi: 10.1097/ALN.0000000000002826. Anesthesiology. 2019. PMID: 31116105 No abstract available.
Abstract
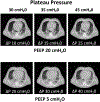
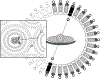
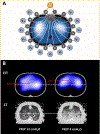
Acute respiratory distress syndrome (ARDS) consists of acute hypoxemic respiratory failure characterized by massive and heterogeneously distributed loss of lung aeration caused by diffuse inflammation and edema present in interstitial and alveolar spaces. It is defined by consensus criteria, which include diffuse infiltrates on chest imaging-either plain radiography or computed tomography. This review will summarize how imaging sciences can inform modern respiratory management of ARDS and continue to increase the understanding of the acutely injured lung. This review also describes newer imaging methodologies that are likely to inform future clinical decision-making and potentially improve outcome. For each imaging modality, this review systematically describes the underlying principles, technology involved, measurements obtained, insights gained by the technique, emerging approaches, limitations, and future developments. Finally, integrated approaches are considered whereby multimodal imaging may impact management of ARDS.
Conflict of interest statement
Figures







References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE: Acute respiratory distress in adults. Lancet 1967; 2: 319–323 - PubMed
-
- Gattinoni L, Pesenti A: ARDS: the non-homogeneous lung; facts and hypotheses. Intensive and Crit Care Digest 1987; 6: 1–4
-
- Elicker BM, Jones KT, Naeger DM, Frank JA: Imaging of Acute Lung Injury. Radiol Clin North Am 2016; 54: 1119–1132 - PubMed
-
- Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M: Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 1987; 136: 730–736 - PubMed
-
- Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ: A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158: 1644–1655 - PubMed

