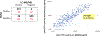Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test
- PMID: 30664078
- PMCID: PMC6456396
- DOI: 10.1097/QAI.0000000000001971
Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test
Abstract
Background: Current pharmacologic adherence monitoring for antiretrovirals involves expensive, labor-intensive liquid chromatography/tandem mass spectrometry (LC-MS/MS)-based methods. Antibody-based assays can monitor and support adherence in real time. We developed a tenofovir (TFV)-based immunoassay and further validated it in a directly observed therapy (DOT) study.
Design: Pharmacologic DOT study of TFV disoproxil fumarate (TDF)/emtricitabine (FTC) administered to HIV-noninfected volunteers.
Methods: The TARGET study provided directly observed TDF 300 mg/FTC 200 mg 7 (high adherence), 4 (moderate), and 2 doses/week (low) to 30 volunteers (10/group) in Thailand, collecting a total of 637 urine samples over 6 weeks of administration and during washout. ELISA measured urine TFV levels by the immunoassay and LC-MS/MS-based concentrations served as the gold standard. A mixed-effects regression model evaluated cutoffs for a point-of-care assay. Performance characteristics of the immunoassay were compared with LC-MS/MS at a chosen cutoff.
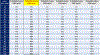
Results: Median TFV levels were 12,000 ng/mL by the immunoassay 1 day after dosing; 5000 ng/mL 2 days after dosing; 1500 ng/mL 3 days after dosing; and below the lower limit of quantification thereafter (≥4 days). An immunoassay cutoff of 1500 ng/mL accurately classified 98% of patients who took a dose 24 hours ago as adherent. The specificity and sensitivity of the immunoassay compared with LC-MS/MS at the 1500 ng/mL cutoff were 99% and 94%; the correlation between TFV levels by the 2 assays was high (0.92, P < 0.00001).
Conclusions: We have developed a novel TFV immunoassay that is highly specific, sensitive, and correlates strongly with LC-MS/MS measurements in a large DOT study. Adherence benchmarks from this DOT study will guide the development of a low-cost rapid point-of-care test for pre-exposure prophylaxis and antiretroviral treatment adherence monitoring and interventions.
Figures