Laminar shear stress alters endothelial KCa2.3 expression in H9c2 cells partially via regulating the PI3K/Akt/p300 axis
- PMID: 30664154
- PMCID: PMC6365081
- DOI: 10.3892/ijmm.2019.4063
Laminar shear stress alters endothelial KCa2.3 expression in H9c2 cells partially via regulating the PI3K/Akt/p300 axis
Abstract
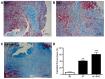
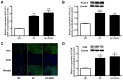
In cardiac tissues, myoblast atrial myocytes continue to be exposed to mechanical forces including shear stress. However, little is known about the effects of shear stress on atrial myocytes, particularly on ion channel function, in association with disease. The present study demonstrated that the Ca2+‑activated K+ channel (KCa)2.3 serves a vital role in regulating arterial tone. As increased intracellular Ca2+ levels and activation of histone acetyltransferase p300 (p300) are early responses to laminar shear stress (LSS) that result in the transcriptional activation of genes, the role of p300 and the phosphoinositide3‑kinase (PI3K)/protein kinase B (Akt) pathway, an intracellular pathway that promotes the growth and proliferation rather than the differentiation of adult cells, in the LSS‑dependent regulation of KCa2.3 in cardiac myoblasts was examined. In cultured H9c2 cells, exposure to LSS (15 dyn/cm2) for 12 h markedly increased KCa2.3 mRNA expression. Inhibiting PI3K attenuated the LSS‑induced increases in the expression and channel activity of KCa2.3, and decreased the phosphorylation levels of p300. The upregulation of these channels was abolished by the inhibition of Akt through decreasing p300 phosphorylation. ChIP assays indicated that p300 was recruited to the promoter region of the KCa2.3 gene. Therefore, the PI3K/Akt/p300 axis serves a crucial role in the LSS‑dependent induction of KCa2.3 expression, by regulating cardiac myoblast function and adaptation to hemodynamic changes. The key novel insights gained from the present study are: i) KCa2.3 was upregulated in patients with atrial fibrillation (AF) and in patients with AF combined with mitral value disease; ii) LSS induced a profound upregulation of KCa2.3 mRNA and protein expression in H9c2 cells; iii) PI3K activation was associated with LSS‑induced upregulation of the KCa2.3 channel; iv) PI3K activation was mediated by PI3K/Akt‑dependent Akt activation; and v) LSS induction of KCa2.3 involved the binding of p300 to transcription factors in the promoter region of the KCa2.3 gene.
Figures



References
-
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. - DOI - PubMed
-
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

