The formation of all-cis-(multi)fluorinated piperidines by a dearomatization-hydrogenation process
- PMID: 30664720
- PMCID: PMC6522351
- DOI: 10.1038/s41557-018-0197-2
The formation of all-cis-(multi)fluorinated piperidines by a dearomatization-hydrogenation process
Abstract
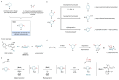
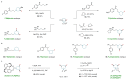
Piperidines and fluorine substituents are both independently indispensable components in pharmaceuticals, agrochemicals and materials. Logically, the incorporation of fluorine atoms into piperidine scaffolds is therefore an area of tremendous potential. However, synthetic approaches towards the formation of these architectures are often impractical. The diastereoselective synthesis of substituted monofluorinated piperidines often requires substrates with pre-defined stereochemistry. That of multifluorinated piperidines is even more challenging, and often needs to be carried out in multistep syntheses. In this report, we describe a straightforward process for the one-pot rhodium-catalysed dearomatization-hydrogenation of fluoropyridine precursors. This strategy enables the formation of a plethora of substituted all-cis-(multi)fluorinated piperidines in a highly diastereoselective fashion through pyridine dearomatization followed by complete saturation of the resulting intermediates by hydrogenation. Fluorinated piperidines with defined axial/equatorial orientation of fluorine substituents were successfully applied in the preparation of commercial drugs analogues. Additionally, fluorinated PipPhos as well as fluorinated ionic liquids were obtained by this dearomatization-hydrogenation process.
Conflict of interest statement
Z.N., C.S. and F.G. are inventors on German patent application 10 2018 104 201.9 held by WWU Muenster that covers the DAH process for the synthesis of all-
Figures

References
-
- Trost BM. Selectivity: a key to synthetic efficiency. Science. 1983;219:245–250. - PubMed
-
- Baran PS, Maimone TJ, Richter JM. Total synthesis of marine natural products without using protecting groups. Nature. 2007;446:404–408. - PubMed
-
- Young IS, Baran PS. Protecting-group-free synthesis as an opportunity for invention. Nat Chem. 2009;1:193–205. - PubMed
-
- Zhao D, Candish L, Paul D, Glorius F. N-Heterocyclic carbenes in asymmetric hydrogenation. ACS Catal. 2016;6:5978–5988.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

