Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy
- PMID: 30664790
- PMCID: PMC6948102
- DOI: 10.1038/s41556-018-0267-0
Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy
Abstract
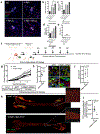
The presence of disseminated tumour cells (DTCs) in bone marrow is predictive of poor metastasis-free survival of patients with breast cancer with localized disease. DTCs persist in distant tissues despite systemic administration of adjuvant chemotherapy. Many assume that this is because the majority of DTCs are quiescent. Here, we challenge this notion and provide evidence that the microenvironment of DTCs protects them from chemotherapy, independent of cell cycle status. We show that chemoresistant DTCs occupy the perivascular niche (PVN) of distant tissues, where they are protected from therapy by vascular endothelium. Inhibiting integrin-mediated interactions between DTCs and the PVN, driven partly by endothelial-derived von Willebrand factor and vascular cell adhesion molecule 1, sensitizes DTCs to chemotherapy. Importantly, chemosensitization is achieved without inducing DTC proliferation or exacerbating chemotherapy-associated toxicities, and ultimately results in prevention of bone metastasis. This suggests that prefacing adjuvant therapy with integrin inhibitors is a viable clinical strategy to eradicate DTCs and prevent metastasis.
Conflict of interest statement
Competing Interests.
The authors declare no competing interests.
Figures






Comment in
-
Therapy resistance beyond cellular dormancy.Nat Cell Biol. 2019 Feb;21(2):117-119. doi: 10.1038/s41556-019-0276-7. Nat Cell Biol. 2019. PMID: 30664787 No abstract available.
References
-
- Karrison TG, Ferguson DJ & Meier P Dormancy of mammary carcinoma after mastectomy. Journal of the National Cancer Institute 91, 80–85 (1999). - PubMed
-
- Braun S et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 18, 80–86 (2000). - PubMed
-
- Janni W et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clinical cancer research : an official journal of the American Association for Cancer Research 17, 2967–2976 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

