Acute AT1R blockade prevents isoproterenol-induced injury in mdx hearts
- PMID: 30664850
- PMCID: PMC6402877
- DOI: 10.1016/j.yjmcc.2019.01.013
Acute AT1R blockade prevents isoproterenol-induced injury in mdx hearts
Abstract
Background: Duchenne muscular dystrophy (DMD) is an X-linked disease characterized by skeletal muscle degeneration and a significant cardiomyopathy secondary to cardiomyocyte damage and myocardial loss. The molecular basis of DMD lies in the absence of the protein dystrophin, which plays critical roles in mechanical membrane integrity and protein localization at the sarcolemma. A popular mouse model of DMD is the mdx mouse, which lacks dystrophin and displays mild cardiac and skeletal pathology that can be exacerbated to advance the disease state. In clinical and pre-clinical studies of DMD, angiotensin signaling pathways have emerged as therapeutic targets due to their adverse influence on muscle remodeling and oxidative stress. Here we aim to establish a physiologically relevant cardiac injury model in the mdx mouse, and determine whether acute blockade of the angiotensin II type 1 receptor (AT1R) may be utilized for prevention of dystrophic injury.
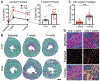
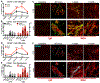
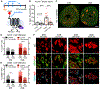
Methods and results: A single IP injection of isoproterenol (Iso, 10 mg/kg) was used to induce cardiac stress and injury in mdx and wild type (C57Bl/10) mice. Mice were euthanized 8 h, 30 h, 1 week, or 1 month following the injection, and hearts were harvested for injury evaluation. At 8 and 30 h post-injury, mdx hearts showed 2.2-fold greater serum cTnI content and 3-fold more extensive injury than wild type hearts. Analysis of hearts 1 week and 1 month after injury revealed significantly higher fibrosis in mdx hearts, with a more robust and longer-lasting immune response compared to wild type hearts. In the 30-hour group, losartan treatment initiated 1 h before Iso injection protected dystrophic hearts from cardiac damage, reducing mdx acute injury area by 2.8-fold, without any significant effect on injury in wild type hearts. However, both wild type and dystrophic hearts showed a 2-fold reduction in the magnitude of the macrophage response to injury 30 h after Iso with losartan.
Conclusions: This work demonstrates that acute blockade of AT1R has the potential for robust injury prevention in a model of Iso-induced dystrophic heart injury. In addition to selectively limiting dystrophic cardiac damage, blocking AT1R may serve to limit the inflammatory nature of the immune response to injury in all hearts. Our findings strongly suggest that earlier adoption of angiotensin receptor blockers in DMD patients could limit myocardial damage and subsequent cardiomyopathy.
Keywords: Angiotensin; Duchenne muscular dystrophy; Dystrophic cardiomyopathy; Fibrosis; Immune infiltration.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures
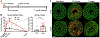

References
-
- Nigro G, Comi LI, Politano L, Bain RJ, The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy, Int J Cardiol. 26 (1990) 271–277. - PubMed
-
- Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM, Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management, Lancet Neurol. 17 (2018) 347–361. doi:10.1016/S1474-4422(18)30025-5. - DOI - PMC - PubMed
-
- McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Groh WJ, Hoffman EP, Judge DP, Kertesz N, Kinnett K, Kirsch R, Metzger JM, Pearson GD, Rafael-Fortney JA, Raman SV, Spurney CF, Targum SL, Wagner KR, Markham LW, and B I. Working Group of the National Heart, Lung, Parent Project Muscular Dystrophy, Contemporary cardiac issues in duchenne muscular dystrophy., Circulation. 131 (2015) 1590–8. doi:10.1161/CIRCULATIONAHA.114.015151. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

