Impaired Annulus Fibrosus Development and Vertebral Fusion Cause Severe Scoliosis in Mice with Deficiency of c-Jun NH2-Terminal Kinases 1 and 2
- PMID: 30664861
- PMCID: PMC6446226
- DOI: 10.1016/j.ajpath.2018.12.010
Impaired Annulus Fibrosus Development and Vertebral Fusion Cause Severe Scoliosis in Mice with Deficiency of c-Jun NH2-Terminal Kinases 1 and 2
Abstract
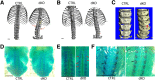
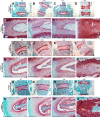
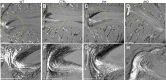
Mitogen-activated protein kinases, including c-Jun NH2-terminal kinase (JNK), play an important role in the development and function of a large variety of tissues. The skeletal phenotype of JNK1 and JNK2 double-knockout (dKO) mice (JNK1fl/flCol2-Cre/JNK2-/-) and control genotypes were analyzed at different embryonic and postnatal stages. JNK1/2 dKO mice displayed a severe scoliotic phenotype beginning during development that was grossly apparent around weaning age. Alcian blue staining at embryonic day 17.5 showed abnormal fusion of the posterior spinal elements. In adult mice, fusion of vertebral bodies and of spinous and transverse processes was noted by micro-computed tomography, Alcian blue/Alizarin red staining, and histology. The long bones developed normally, and histologic sections of growth plate and articular cartilage revealed no significant abnormalities. Histologic sections of the vertebral column at embryonic days 15.5 and 17.5 revealed an abnormal organization of the annulus fibrosus in the dKOs, with chondrocyte-like cells and fusion of dorsal processes. Spinal sections in 10-week-old dKO mice showed replacement of intervertebral disk structures (annulus fibrosus and nucleus pulposus) by cartilage and bone tissues, with cells staining for markers of hypertrophic chondrocytes, including collagen X and runt-related transcription factor 2. These findings demonstrate a requirement for both JNK1 and JNK2 in the normal development of the axial skeleton. Loss of JNK signaling results in abnormal endochondral bone formation and subsequent severe scoliosis.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures










References
-
- Bobick B.E., Kulyk W.M. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Res C Embryo Today. 2008;84:131–154. - PubMed
-
- Clancy R., Rediske J., Koehne C., Stoyanovsky D., Amin A., Attur M., Iyama K., Abramson S.B. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

