Metal Toxicity Links to Alzheimer's Disease and Neuroinflammation
- PMID: 30664867
- PMCID: PMC6475603
- DOI: 10.1016/j.jmb.2019.01.018
Metal Toxicity Links to Alzheimer's Disease and Neuroinflammation
Abstract
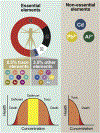
As the median age of the population increases, the number of individuals with Alzheimer's disease (AD) and the associated socio-economic burden are predicted to worsen. While aging and inherent genetic predisposition play major roles in the onset of AD, lifestyle, physical fitness, medical condition, and social environment have emerged as relevant disease modifiers. These environmental risk factors can play a key role in accelerating or decelerating disease onset and progression. Among known environmental risk factors, chronic exposure to various metals has become more common among the public as the aggressive pace of anthropogenic activities releases excess amount of metals into the environment. As a result, we are exposed not only to essential metals, such as iron, copper, zinc and manganese, but also to toxic metals including lead, aluminum, and cadmium, which perturb metal homeostasis at the cellular and organismal levels. Herein, we review how these metals affect brain physiology and immunity, as well as their roles in the accumulation of toxic AD proteinaceous species (i.e., β-amyloid and tau). We also discuss studies that validate the disruption of immune-related pathways as an important mechanism of toxicity by which metals can contribute to AD. Our goal is to increase the awareness of metals as players in the onset and progression of AD.
Keywords: dementia; environment; neurodegeneration; tau; β-amyloid.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
-
- United Nations, Department of Economic and Social Affairs, Population Division (2017). World Population Prospects: The 2017 Revision, Key Findings and Advance Tables.
-
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L & Mukadam N (2017). Dementia prevention, intervention, and care. Lancet. 390,2673–734. - PubMed
-
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, Mcdade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S & Morris JC (2012). Clinical and biomarker changes in dominantly inherited alzheimer’s disease. N Engl J Med. 367,795–804. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

