Glucocorticoids promote the development of azoxymethane and dextran sulfate sodium-induced colorectal carcinoma in mice
- PMID: 30665389
- PMCID: PMC6341596
- DOI: 10.1186/s12885-019-5299-8
Glucocorticoids promote the development of azoxymethane and dextran sulfate sodium-induced colorectal carcinoma in mice
Abstract
Background: Stress has been suggested as a promoter of tumor growth and development. Glucocorticoids (GCs) are the main stress hormones and widely prescribed as drugs. However, the effect of GCs on the development and progression of colorectal carcinoma (CRC) is unclear.
Methods: We evaluated the effect of corticosterone (CORT) on azoxymethane and dextran sulfate sodium (AOM/DSS)-induced carcinogenesis in the colorectum of C57BL/6 strain mice. Plasma level of CORT was detected by radioimmunoassay. The expression of proliferation markers (Ki-67 and PCNA), nuclear factor (NF)-κB p65 and phosphoto-p65 (P-p65), as well as cyclooxygenase (COX)-2 were determined by immunohistochemistry. Inflammation in colorectum was evaluated by histopathology.
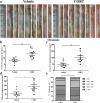
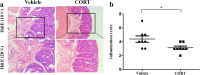
Results: CORT feeding in drinking water of mice not only significantly elevated plasma CORT concentration, but also significantly increased the incidence and neoplasms burden (number and size of neoplasms) in colorectum. CORT also significant enhanced the expression of cell proliferation marker (Ki-67 and PCNA), NF-κB p65 and P-p65 as well as COX-2 in colorectal neoplasm of AOM/DSS-treated mice.
Conclusion: In this study, we have found for the first time that CORT at stress level potentially promotes the growth and development of AOM/DSS-induced colorectal adenoma and carcinoma in mice. Up-regulation of NF-κB and COX-2 may be involved in the promoting effect of CORT.
Keywords: Azoxymethane/dextran sodium sulfate; Colorectal carcinoma; Corticosterone; Nuclear factor-κB; Tumor development.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Animal Care and Use Committee of Second Military Medical University (Shanghai, China) and performed in compliance with the University’s Guidelines for the Care and Use of Laboratory Animals (reference number: 20131015001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Gundisch S, Boeckeler E, Behrends U, Amtmann E, Ehrhardt H, Jeremias I. Glucocorticoids augment survival and proliferation of tumor cells. Anticancer Res. 2012;32(10):4251–4261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

