NK cells specifically TCR-dressed to kill cancer cells
- PMID: 30665853
- PMCID: PMC6413353
- DOI: 10.1016/j.ebiom.2019.01.031
NK cells specifically TCR-dressed to kill cancer cells
Abstract
Background: Adoptive T-cell transfer of therapeutic TCR holds great promise to specifically kill cancer cells, but relies on modifying the patient's own T cells ex vivo before injection. The manufacturing of T cells in a tailor-made setting is a long and expensive process which could be resolved by the use of universal cells. Currently, only the Natural Killer (NK) cell line NK-92 is FDA approved for universal use. In order to expand their recognition ability, they were equipped with Chimeric Antigen Receptors (CARs). However, unlike CARs, T-cell receptors (TCRs) can recognize all cellular proteins, which expand NK-92 recognition to the whole proteome.
Methods: We herein genetically engineered NK-92 to express the CD3 signaling complex, and showed that it rendered them able to express a functional TCR. Functional assays and in vivo efficacy were used to validate these cells.
Findings: This is the first demonstration that a non-T cell can exploit TCRs. This TCR-redirected cell line, termed TCR-NK-92, mimicked primary T cells phenotypically, metabolically and functionally, but retained its NK cell effector functions. Our results demonstrate a unique manner to indefinitely produce TCR-redirected lymphocytes at lower cost and with similar therapeutic efficacy as redirected T cells.
Interpretation: These results suggest that an NK cell line could be the basis for an off-the-shelf TCR-based cancer immunotherapy solution. FUND: This work was supported by the Research Council of Norway (#254817), South-Eastern Norway Regional Health Authority (#14/00500-79), by OUS-Radiumhospitalet (Gene Therapy program) and the department of Oncology at the University of Lausanne.
Keywords: Immunotherapy; Natural killer; T cell; TCR.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
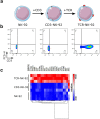

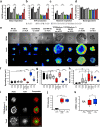
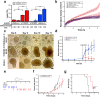

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

