Nanotubular TiO2 regulates macrophage M2 polarization and increases macrophage secretion of VEGF to accelerate endothelialization via the ERK1/2 and PI3K/AKT pathways
- PMID: 30666106
- PMCID: PMC6330985
- DOI: 10.2147/IJN.S188439
Nanotubular TiO2 regulates macrophage M2 polarization and increases macrophage secretion of VEGF to accelerate endothelialization via the ERK1/2 and PI3K/AKT pathways
Abstract
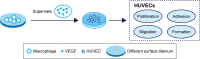
Background: Macrophages play important roles in the immune response to, and successful implantation of, biomaterials. Titanium nanotubes are considered promising heart valve stent materials owing to their effects on modulation of macrophage behavior. However, the effects of nanotube-regulated macrophages on endothelial cells, which are essential for stent endothelialization, are unknown. Therefore, in this study we evaluated the inflammatory responses of endothelial cells to titanium nanotubes prepared at different voltages.
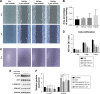
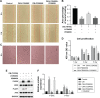
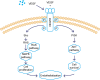
Methods and results: In this study we used three different voltages (20, 40, and 60 V) to produce titania nanotubes with three different diameters by anodic oxidation. The state of macrophages on the samples was assessed, and the supernatants were collected as conditioned media (CM) to stimulate human umbilical vein endothelial cells (HUVECs), with pure titanium as a control group. The results indicated that titanium dioxide (TiO2) nanotubes induced macrophage polarization toward the anti-inflammatory M2 state and increased the expression of arginase-1, mannose receptor, and interleukin 10. Further mechanistic analysis revealed that M2 macrophage polarization controlled by the TiO2 nanotube surface activated the phosphatidylinositol 3-kinase/AKT and extracellular signal-regulated kinase 1/2 pathways through release of vascular endothelial growth factor to influence endothelialization.
Conclusion: Our findings expanded our understanding of the complex influence of nanotubes in implants and the macrophage inflammatory response. Furthermore, CM generated from culture on the TiO2 nanotube surface may represent an integrated research model for studying the interactions of two different cell types and may be a promising approach for accelerating stent endothelialization through immunoregulation.
Keywords: TiO2 nanotubes; axitinib; conditioned medium; endothelial cells; stent implant.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS) Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33(19):2451–2496. - PubMed
-
- de Mel A, Jell G, Stevens MM, Seifalian AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9(11):2969–2979. - PubMed
-
- Li G, Yang P, Qin W, Maitz MF, Zhou S, Huang N. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials. 2011;32(21):4691–4703. - PubMed
-
- Rossetti FF, Bally M, Michel R, Textor M, Reviakine I. Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir. 2005;21(14):6443–6450. - PubMed
-
- Kulkarni M, Mazare A, Gongadze E, et al. Titanium nanostructures for biomedical applications. Nanotechnology. 2015;26(6):062002. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

