Hippocampal Neurogenesis Reduces the Dimensionality of Sparsely Coded Representations to Enhance Memory Encoding
- PMID: 30666194
- PMCID: PMC6330828
- DOI: 10.3389/fncom.2018.00099
Hippocampal Neurogenesis Reduces the Dimensionality of Sparsely Coded Representations to Enhance Memory Encoding
Abstract
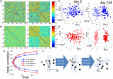
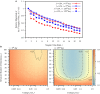
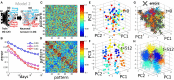
Adult neurogenesis in the hippocampal dentate gyrus (DG) of mammals is known to contribute to memory encoding in many tasks. The DG also exhibits exceptionally sparse activity compared to other systems, however, whether sparseness and neurogenesis interact during memory encoding remains elusive. We implement a novel learning rule consistent with experimental findings of competition among adult-born neurons in a supervised multilayer feedforward network trained to discriminate between contexts. From this rule, the DG population partitions into neuronal ensembles each of which is biased to represent one of the contexts. This corresponds to a low dimensional representation of the contexts, whereby the fastest dimensionality reduction is achieved in sparse models. We then modify the rule, showing that equivalent representations and performance are achieved when neurons compete for synaptic stability rather than neuronal survival. Our results suggest that competition for stability in sparse models is well-suited to developing ensembles of what may be called memory engram cells.
Keywords: dimensionality reduction; feed-forward neural network; hippocampus; neuromorphic computing; pattern separation; synaptic plasticity; synaptic pruning; synaptic turnover.
Figures




References
LinkOut - more resources
Full Text Sources

