The Roles of Mitochondrial SIRT4 in Cellular Metabolism
- PMID: 30666234
- PMCID: PMC6330279
- DOI: 10.3389/fendo.2018.00783
The Roles of Mitochondrial SIRT4 in Cellular Metabolism
Abstract
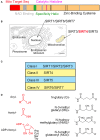
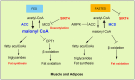
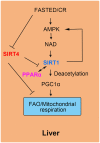
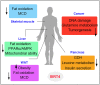
Sirtuins comprise a family of nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacylases that regulate the life span, aging, and metabolism. Seven sirtuin family members (SIRT1-7) have been identified in mammals, including humans. Despite the indispensable role of mitochondrial sirtuin 4 (SIRT4) in metabolic regulation, the primary enzymatic activity of SIRT4 remains enigmatic. SIRT4 possesses ADP-ribosyltransferase, lipoamidase and deacylase activities. Interestingly, the enzymatic activities and substrates of SIRT4 vary in different tissues and cells. SIRT4 inhibits insulin secretion in pancreatic β cells and regulates insulin sensitivity as a deacylase in the pancreas. SIRT4 represses fatty acid oxidation (FAO) in muscle and liver cells differently. SIRT4 has also been identified as a mitochondrial-localized tumor suppressor. A comprehensive understanding of the enzymology of SIRT4 in metabolism is essential for developing novel therapeutic agents for human metabolic diseases. This review will update the roles of SIRT4 in cellular and organismal metabolic homeostasis.
Keywords: SIRT4; enzymatic activities; fatty acid metabolism; insulin secretion; mitochondrial; tumor suppressor.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

