Engineering and Design of Chimeric Antigen Receptors
- PMID: 30666307
- PMCID: PMC6330382
- DOI: 10.1016/j.omtm.2018.12.009
Engineering and Design of Chimeric Antigen Receptors
Abstract
T cells engineered with chimeric antigen receptors (CARs) have emerged as a potent new class of therapeutics for cancer, based on their remarkable potency in blood cancers. Since the first clinical reports of their efficacy emerged 7 years ago, investigators have focused on the mechanisms and properties that make CARs effective or toxic, and their effects on T cell biology. Novel CAR designs coupled with improvements in gene transfer technology, incorporating advances in gene editing, have the potential to increase access to engineered cell therapies, as well as improve their potency in solid tumors.
Keywords: CAR T cells; cancer; chimeric antigen receptors; immunotherapy.
Figures
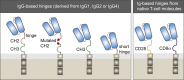
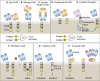
References
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

