Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn's disease: a meta-analysis
- PMID: 30666565
- PMCID: PMC6394648
- DOI: 10.1007/s12519-018-0204-0
Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn's disease: a meta-analysis
Abstract
Background: Many studies have examined the effects of exclusive enteral nutrition (EEN) in children with Crohn's disease (CD), but corticosteroids are considered a superior therapy and are frequently used in China. This meta-analysis aims to compare the efficacy of EEN with corticosteroids in treating pediatric CD.
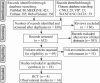
Methods: A comprehensive retrieval from medical databases, including PubMed, EMBASE, MEDLINE, Web of Science, Wanfang data, VIP and CNKI, was performed using the search terms "diet therapy", "exclusive enteral nutrition", "Crohn's disease", "inflammatory bowel diseases", "child" and "pediatrics" from January 1990 to April 2017.
Results: We included 18 studies from 1329 identified sources in this meta-analysis. EEN was as effective as corticosteroids in inducing remission rate of children suffering from CD (OR = 1.35; 95% CI 0.90, 2.10; P = 0.14). Nevertheless, patients who received EEN were more likely to achieve both endoscopic mucosal healing (OR = 5.24; 95% CI 2.06, 13.37; P = 0.0005) and histological mucosal healing (OR = 4.78; 95% CI 1.89, 12.08; P = 0.0009) than those who received corticosteroids; the Pediatric Crohn's Disease Activity Index was lower [mean difference (MD) = - 3.67; 95% CI - 4.91, - 2.43] and weight gain was higher (MD = 1.92; 95% CI 0.02, 3.83; P = 0.05) in those patients who received EEN than in those who received corticosteroids. No difference was found in relapse rate (OR = 0.57; 95% CI 0.25, 1.29; P = 0.18), height for age or body mass index between the patients treated with EEN and corticosteroids at the 1-year end point.
Conclusions: This meta-analysis reveals that there is no significant difference between EEN and corticosteroids in the efficacy of inducing remission rate of CD in a pediatric population, but EEN is superior to corticosteroids in improving short-term mucosal inflammation and reducing the PCDAI index.
Keywords: Children; Corticosteroids; Crohn’s disease; Exclusive enteral nutrition; Inflammatory bowel disease; Nutrition.
Conflict of interest statement
Ethical approval
None.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

