Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration
- PMID: 30666821
- PMCID: PMC6525555
- DOI: 10.1002/sctm.18-0141
Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration
Abstract
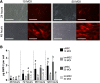
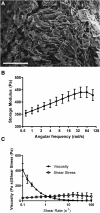
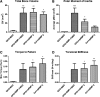
Bone morphogenetic protein 2 (BMP-2)-loaded collagen sponges remain the clinical standard for treatment of large bone defects when there is insufficient autograft, despite associated complications. Recent efforts to negate comorbidities have included biomaterials and gene therapy approaches to extend the duration of BMP-2 release and activity. In this study, we compared the collagen sponge clinical standard to chondroitin sulfate glycosaminoglycan (CS-GAG) scaffolds as a delivery vehicle for recombinant human BMP-2 (rhBMP-2) and rhBMP-2 expression via human BMP-2 gene inserted into mesenchymal stem cells (BMP-2 MSC). We demonstrated extended release of rhBMP-2 from CS-GAG scaffolds compared to their collagen sponge counterparts, and further extended release from CS-GAG gels seeded with BMP-2 MSC. When used to treat a challenging critically sized femoral defect model in rats, both rhBMP-2 and BMP-2 MSC in CS-GAG induced comparable bone formation to the rhBMP-2 in collagen sponge, as measured by bone volume, strength, and stiffness. We conclude that CS-GAG scaffolds are a promising delivery vehicle for controlling the release of rhBMP-2 and to mediate the repair of critically sized segmental bone defects. Stem Cells Translational Medicine 2019;8:575-585.
Keywords: Bone morphogenetic protein-2; Chondroitin sulfate glycosaminoglycan; Genetic therapy; Mesenchymal stromal cells; Osteogenesis.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures



References
-
- Greenwald AS, Boden SD, Goldberg VM et al. Bone‐graft substitutes: Facts, fictions, and applications. J Bone Joint Surg Am 2001, 83‐A (suppl 2; Pt 2):98–103. - PubMed
-
- Toolan BC. Current concepts review: Orthobiologics. Foot Ankle Int. 2006;27(7):561–566. - PubMed
-
- De Long WG, Einhorn TA, Koval K et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. J Bone Joint Surg Am 2007;89:649–658. - PubMed
-
- Laurencin C, Khan Y, El‐Amin SF. Bone graft substitutes. Expert Rev Med Devices 2006;3:49–57. - PubMed
-
- Desai BM. Osteobiologics. Am J Orthop (Belle Mead NJ) 2007;36 (suppl 4):8–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

