Efficacy and Safety of Belimumab and Azathioprine for Maintenance of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Controlled Study
- PMID: 30666823
- PMCID: PMC6593987
- DOI: 10.1002/art.40802
Efficacy and Safety of Belimumab and Azathioprine for Maintenance of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Controlled Study
Abstract
Objective: To evaluate the safety and efficacy of belimumab as adjunctive therapy to maintain remission in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
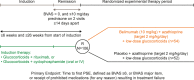
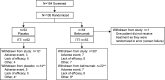
Methods: In this multicenter, double-blind, placebo-controlled study, patients with AAV (ages ≥18 years) were randomized 1:1 to receive azathioprine (2 mg/kg/day), low-dose oral glucocorticoids (≤10 mg/day), and either intravenous belimumab (10 mg/kg) or placebo, following remission induction with rituximab or cyclophosphamide along with glucocorticoids. The primary end point was time to first protocol-specified event (PSE), with first PSE defined as a Birmingham Vasculitis Activity Score (BVAS) of ≥6, presence of ≥1 major BVAS item, or receipt of prohibited medications for any reason, resulting in treatment failure (adjusted for ANCA type [proteinase 3 (PR3) or myeloperoxidase (MPO)], disease stage at induction, and induction regimen). Vasculitis relapse was defined as the PSE of either a BVAS activity score of ≥6 or receipt of prohibited medications for vasculitis. Changes in treatment practice led to truncation of the study population from ~300 patients to ~100 patients.
Results: The intent-to-treat population totaled 105 patients with AAV, of whom 52 (40 with PR3-ANCAs, 12 with MPO-ANCAs) received placebo and 53 (41 with PR3-ANCAs, 12 with MPO-ANCAs) received belimumab; 27 of the patients were in rituximab-induced disease remission, while 78 were in cyclophosphamide-induced disease remission at baseline. Compared with placebo, treatment with belimumab did not reduce the risk of a PSE (adjusted hazard ratio [HR] 1.07, 95% confidence interval [95% CI] 0.44-2.59; P = 0.884) or vasculitis relapse (adjusted HR 0.88, 95% CI 0.29-2.65; P = 0.821). The overall rate of PSEs was low (11 [21.2%] of 52 patients receiving placebo, 10 [18.9%] of 53 patients receiving belimumab). Vasculitis relapse in the placebo group (n = 8) occurred independent of the induction regimen, disease stage, or ANCA type. All vasculitis relapses in the belimumab group (n = 6) occurred in patients who had PR3-ANCA-associated vasculitis with cyclophosphamide-induced disease remission. Adverse events occurred in 49 (92.5%) of 53 patients receiving belimumab and 43 (82.7%) of 52 patients receiving placebo, with no new safety concerns.
Conclusion: Belimumab plus azathioprine and glucocorticoids for the maintenance of remission in AAV did not reduce the risk of relapse.
Trial registration: ClinicalTrials.gov NCT01663623.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

References
-
- Cartin‐Ceba R, Peikert T, Specks U. Pathogenesis of ANCA‐associated vasculitis. Curr Rheumatol Rep 2012;14:481–93. - PubMed
-
- Jennette JC, Falk RJ. Small‐vessel vasculitis. N Engl J Med 1997;337:1512–23. - PubMed
-
- Yates M, Watts R, Bajema I, Cid M, Crestani B, Hauser T, et al. EULAR/ERA‐EDTA recommendations for the management of ANCA‐associated vasculitis. Ann Rheum Dis 2016;75:1583–94. - PubMed
-
- Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA‐associated vasculitis. N Engl J Med 2014;371:1771–80. - PubMed
-
- Turner‐Stokes T, Sandhu E, Pepper RJ, Stolagiewicz NE, Ashley C, Dinneen D, et al. Induction treatment of ANCA‐associated vasculitis with a single dose of rituximab. Rheumatology (Oxford) 2014;53:1395–403. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

