Differential Shedding and Antibody Kinetics of Zika and Chikungunya Viruses, Brazil
- PMID: 30666934
- PMCID: PMC6346451
- DOI: 10.3201/eid2502.180166
Differential Shedding and Antibody Kinetics of Zika and Chikungunya Viruses, Brazil
Abstract
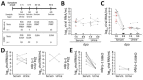
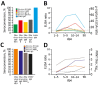
In seroconversion panels obtained from patients from Brazil, diagnostic testing for Zika virus infection was improved by combining multiple antibody isotypes, techniques, and antigens, but sensitivity remained suboptimal. In contrast, chikungunya virus diagnostic testing was unambiguous. Recurrent recent arbovirus infections suggested by serologic data and unspecific symptoms highlight the need for exhaustive virologic testing.
Keywords: Brazil; Zika virus; arboviruses; chikungunya virus; diagnosis; flaviviruses; real time RT-PCR; serology; vector-borne infections; viruses.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

