Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells
- PMID: 30667095
- PMCID: PMC6590475
- DOI: 10.1002/glia.23579
Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells
Abstract
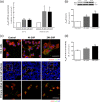
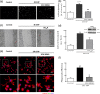
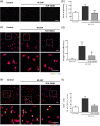
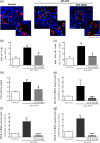
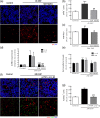
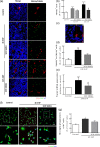
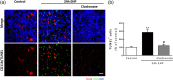
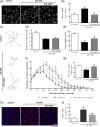
Glaucoma is a retinal degenerative disease characterized by the loss of retinal ganglion cells and damage of the optic nerve. Recently, we demonstrated that antagonists of adenosine A2A receptor (A2A R) control retinal inflammation and afford protection to rat retinal cells in glaucoma models. However, the precise contribution of microglia to retinal injury was not addressed, as well as the effect of A2A R blockade directly in microglia. Here we show that blocking microglial A2A R prevents microglial cell response to elevated pressure and it is sufficient to protect retinal cells from elevated pressure-induced death. The A2A R antagonist SCH 58261 or the knockdown of A2A R expression with siRNA in microglial cells prevented the increase in microglia response to elevated hydrostatic pressure. Furthermore, in retinal neural cell cultures, the A2A R antagonist decreased microglia proliferation, as well as the expression and release of pro-inflammatory mediators. Microglia ablation prevented neural cell death triggered by elevated pressure. The A2A R blockade recapitulated the effects of microglia depletion, suggesting that blocking A2A R in microglia is able to control neurodegeneration in glaucoma-like conditions. Importantly, in human organotypic retinal cultures, A2A R blockade prevented the increase in reactive oxygen species and the morphological alterations in microglia triggered by elevated pressure. These findings place microglia as the main contributors for retinal cell death during elevated pressure and identify microglial A2A R as a therapeutic target to control retinal neuroinflammation and prevent neural apoptosis elicited by elevated pressure.
Keywords: adenosine A2A receptors; glaucoma; microglia; neurodegeneration; neuroinflammation.
© 2019 The Authors. Glia published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure.J Neuroinflammation. 2015 Jun 10;12:115. doi: 10.1186/s12974-015-0333-5. J Neuroinflammation. 2015. PMID: 26054642 Free PMC article.
-
Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury.Transl Res. 2016 Mar;169:112-28. doi: 10.1016/j.trsl.2015.11.005. Epub 2015 Nov 23. Transl Res. 2016. PMID: 26685039
-
Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage.Cell Death Dis. 2017 Oct 5;8(10):e3065. doi: 10.1038/cddis.2017.451. Cell Death Dis. 2017. PMID: 28981089 Free PMC article.
-
Role of microglia adenosine A(2A) receptors in retinal and brain neurodegenerative diseases.Mediators Inflamm. 2014;2014:465694. doi: 10.1155/2014/465694. Epub 2014 Jul 16. Mediators Inflamm. 2014. PMID: 25132733 Free PMC article. Review.
-
Therapeutic Opportunities for Caffeine and A2A Receptor Antagonists in Retinal Diseases.Ophthalmic Res. 2016;55(4):212-8. doi: 10.1159/000443893. Epub 2016 Mar 10. Ophthalmic Res. 2016. PMID: 26959995 Review.
Cited by
-
Istradefylline protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin antitumor effects.J Clin Invest. 2022 Nov 15;132(22):e152924. doi: 10.1172/JCI152924. J Clin Invest. 2022. PMID: 36377661 Free PMC article.
-
Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading.Biomolecules. 2021 May 21;11(6):770. doi: 10.3390/biom11060770. Biomolecules. 2021. PMID: 34063832 Free PMC article. Review.
-
The adenosine A2A receptor antagonist KW6002 distinctly regulates retinal ganglion cell morphology during postnatal development and neonatal inflammation.Front Pharmacol. 2022 Dec 16;13:1082997. doi: 10.3389/fphar.2022.1082997. eCollection 2022. Front Pharmacol. 2022. PMID: 36588710 Free PMC article.
-
The Role of Neuroinflammation in Glaucoma: An Update on Molecular Mechanisms and New Therapeutic Options.Front Neurol. 2021 Feb 4;11:612422. doi: 10.3389/fneur.2020.612422. eCollection 2020. Front Neurol. 2021. PMID: 33613418 Free PMC article. Review.
-
Blockade of Adenosine A2A Receptor Protects Photoreceptors after Retinal Detachment by Inhibiting Inflammation and Oxidative Stress.Oxid Med Cell Longev. 2020 Jul 2;2020:7649080. doi: 10.1155/2020/7649080. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32714489 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

