A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI
- PMID: 30667329
- PMCID: PMC6394788
- DOI: 10.1148/radiol.2018181278
A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI
Abstract
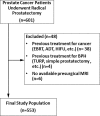
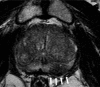
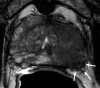
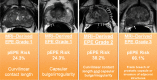
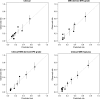
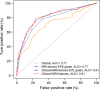
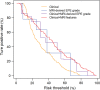
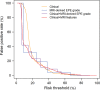
Purpose To evaluate MRI features associated with pathologically defined extraprostatic extension (EPE) of prostate cancer and to propose an MRI grading system for pathologic EPE. Materials and Methods In this prospective study, consecutive male study participants underwent preoperative 3.0-T MRI from June 2007 to March 2017 followed by robotic-assisted laparoscopic radical prostatectomy. An MRI-based EPE grading system was defined as follows: curvilinear contact length of 1.5 cm or capsular bulge and irregularity were grade 1, both features were grade 2, and frank capsular breach were grade 3. Multivariable logistic regression and decision curve analyses were performed to compare the MRI grade model and clinical parameters (prostate-specific antigen, Gleason score) for pathologic EPE prediction by using the area under the receiver operating characteristic curve (AUC) value. Results Among 553 study participants, the mean age was 60 years ± 8 (standard deviation); the median prostate-specific antigen value was 6.3 ng/mL. A total of 125 of 553 (22%) participants had pathologic EPE at radical prostatectomy. Detection of pathologic EPE, defined as number of pathologic EPEs divided by number of participants with individual MRI features, was as follows: curvilinear contact length, 88 of 208 (42%); capsular bulge and irregularity, 78 of 175 (45%); and EPE visible at MRI, 37 of 56 (66%). For MRI, grades 1, 2, and 3 for detection of pathologic EPE were 18 of 74 (24%), 39 of 102 (38%), and 37 of 56 (66%), respectively. Clinical features plus the MRI-based EPE grading system (prostate-specific antigen, International Society of Urological Pathology stage, MRI grade) predicted pathologic EPE better than did MRI grade alone (AUC, 0.81 vs 0.77, respectively; P < .001). Conclusion Higher MRI-based extraprostatic extension (EPE) grading categories were associated with a greater risk of pathologic EPE. Clinical features plus MRI grading had the highest diagnostic performance for prediction of pathologic EPE. © RSNA, 2019 Online supplemental material is available for this article. See also the editorial by Eberhardt in this issue.
Figures





Comment in
-
Local Staging of Prostate Cancer with MRI: A Need for Standardization.Radiology. 2019 Mar;290(3):720-721. doi: 10.1148/radiol.2019182943. Epub 2019 Jan 22. Radiology. 2019. PMID: 30667328 No abstract available.
-
Re: A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI.J Urol. 2019 Sep;202(3):440-441. doi: 10.1097/JU.0000000000000371. Epub 2019 Aug 8. J Urol. 2019. PMID: 31166838 No abstract available.
References
-
- Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002;167(2 Pt 1):528–534. - PubMed
-
- Mikel Hubanks J, Boorjian SA, Frank I, et al. The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol Oncol 2014;32(1):26.e1–26.e7. - PubMed
-
- Tollefson MK, Karnes RJ, Rangel LJ, Bergstralh EJ, Boorjian SA. The impact of clinical stage on prostate cancer survival following radical prostatectomy. J Urol 2013;189(5):1707–1712. - PubMed
-
- Wheeler TM, Dillioglugil O, Kattan MW, et al. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1-2 prostate cancer. Hum Pathol 1998;29(8):856–862. - PubMed
-
- Roethke MC, Lichy MP, Kniess M, et al. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J Urol 2013;31(5):1111–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

