Pharmacological Treatments for Neonatal Abstinence Syndrome: A Systematic Review and Network Meta-analysis
- PMID: 30667476
- PMCID: PMC6439896
- DOI: 10.1001/jamapediatrics.2018.5044
Pharmacological Treatments for Neonatal Abstinence Syndrome: A Systematic Review and Network Meta-analysis
Abstract
Importance: Incidence of neonatal abstinence syndrome is rising rapidly, and optimal pharmacotherapy may meaningfully reduce length of treatment.
Objective: To compare pharmacological therapies for neonatal abstinence syndrome.
Data sources: Systematic review and network meta-analysis of Medline (1946-June 2018), Embase (1974-June 2018), Cochrane CENTRAL (1966-June 2018), Web of Science (1900-June 2018), and ClinicalTrials.gov (June 2018).
Study selection: Randomized clinical trials of pharmacological treatments for neonatal abstinence syndrome alone or in combination with adjuvant treatments. Abstract, title, and full-text screening were conducted independently by 2 reviewers (T.D. and C.G.).
Data extraction and synthesis: Data extraction was conducted independently by 2 reviewers (T.D. and C.G.) according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)-Network Meta-Analyses guidelines. Quality was assessed with the Cochrane Risk of Bias tool and data were pooled with fixed-effect models as a result of the low number of trials that were included in the analysis.
Main outcomes and measures: The primary outcome was the length of treatment. The length of stay, need for adjuvant therapy, and adverse events were considered as secondary outcomes.
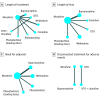
Results: Eighteen trials (N = 1072) were eligible for inclusion. The treatments that were included in the length of treatment analysis were buprenorphine, clonidine, diluted tincture of opium and clonidine, diluted tincture of opium, morphine, methadone, and phenobarbital. Sublingual buprenorphine was considered the optimal treatment for a reduction in the length of treatment (days: mean difference vs morphine, -12.75 [95% CI, -17.97 to -7.58]; median rank, 1 [3-1]) and length of stay (days: mean difference vs morphine, -11.43 [95% CI, -16.95 to -5.82]; median rank, 1 [3-1]) but not the need for adjuvant treatment (odds ratio vs morphine, 1.23 [95% CI, 0.46-3.44]; median rank, 3 [5-1]). The results were robust to bias but sensitive to imprecision.
Conclusions and relevance: The current evidence suggests that buprenorphine is the optimal treatment for neonatal abstinence treatment, but limitations are considerable and wide-scale adoption requires a large multisite trial. Morphine, which is considered standard of care in most hospitals, was the lowest-ranked opioid for length of treatment and length of stay.
Conflict of interest statement
Figures


Comment in
-
Pharmacologic Treatment for Neonatal Abstinence Syndrome: Which Medication Is Best?JAMA Pediatr. 2019 Mar 1;173(3):221-223. doi: 10.1001/jamapediatrics.2018.5029. JAMA Pediatr. 2019. PMID: 30667482 No abstract available.
References
-
- Veritas Health Innovation Covidence systematic review software. http://www.covidence.org. Accessed September 20, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

