Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial
- PMID: 30667502
- PMCID: PMC6440226
- DOI: 10.1001/jama.2018.20579
Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial
Erratum in
-
Revised Dietary Intake Estimates for Protein and Fat.JAMA. 2019 Aug 6;322(5):469. doi: 10.1001/jama.2019.10532. JAMA. 2019. PMID: 31386114 Free PMC article. No abstract available.
Abstract
Importance: Pediatric guidelines for the management of nonalcoholic fatty liver disease (NAFLD) recommend a healthy diet as treatment. Reduction of sugary foods and beverages is a plausible but unproven treatment.
Objective: To determine the effects of a diet low in free sugars (those sugars added to foods and beverages and occurring naturally in fruit juices) in adolescent boys with NAFLD.
Design, setting, and participants: An open-label, 8-week randomized clinical trial of adolescent boys aged 11 to 16 years with histologically diagnosed NAFLD and evidence of active disease (hepatic steatosis >10% and alanine aminotransferase level ≥45 U/L) randomized 1:1 to an intervention diet group or usual diet group at 2 US academic clinical research centers from August 2015 to July 2017; final date of follow-up was September 2017.
Interventions: The intervention diet consisted of individualized menu planning and provision of study meals for the entire household to restrict free sugar intake to less than 3% of daily calories for 8 weeks. Twice-weekly telephone calls assessed diet adherence. Usual diet participants consumed their regular diet.
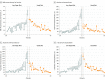
Main outcomes and measures: The primary outcome was change in hepatic steatosis estimated by magnetic resonance imaging proton density fat fraction measurement between baseline and 8 weeks. The minimal clinically important difference was assumed to be 4%. There were 12 secondary outcomes, including change in alanine aminotransferase level and diet adherence.
Results: Forty adolescent boys were randomly assigned to either the intervention diet group or the usual diet group (20 per group; mean [SD] age, 13.0 [1.9] years; most were Hispanic [95%]) and all completed the trial. The mean decrease in hepatic steatosis from baseline to week 8 was significantly greater for the intervention diet group (25% to 17%) vs the usual diet group (21% to 20%) and the adjusted week 8 mean difference was -6.23% (95% CI, -9.45% to -3.02%; P < .001). Of the 12 prespecified secondary outcomes, 7 were null and 5 were statistically significant including alanine aminotransferase level and diet adherence. The geometric mean decrease in alanine aminotransferase level from baseline to 8 weeks was significantly greater for the intervention diet group (103 U/L to 61 U/L) vs the usual diet group (82 U/L to 75 U/L) and the adjusted ratio of the geometric means at week 8 was 0.65 U/L (95% CI, 0.53 to 0.81 U/L; P < .001). Adherence to the diet was high in the intervention diet group (18 of 20 reported intake of <3% of calories from free sugar during the intervention). There were no adverse events related to participation in the study.
Conclusions and relevance: In this study of adolescent boys with NAFLD, 8 weeks of provision of a diet low in free sugar content compared with usual diet resulted in significant improvement in hepatic steatosis. However, these findings should be considered preliminary and further research is required to assess long-term and clinical outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT02513121.
Conflict of interest statement
Figures
Comment in
-
Low Free Sugar Diet in Adolescents With Nonalcoholic Fatty Liver Disease.JAMA. 2019 Jun 25;321(24):2468-2469. doi: 10.1001/jama.2019.5141. JAMA. 2019. PMID: 31237631 No abstract available.
-
Too Much Sugar-The Not-So-Sweet Reality of Its Impact on Our Health.Hepatology. 2020 Jan;71(1):377-379. doi: 10.1002/hep.30910. Epub 2019 Dec 20. Hepatology. 2020. PMID: 31446629 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

