Efficacy and safety of 0.6% sodium alginate solution in endoscopic submucosal dissection for esophageal and gastric neoplastic lesion: A randomized controlled study
- PMID: 30667557
- PMCID: PMC6850280
- DOI: 10.1111/den.13352
Efficacy and safety of 0.6% sodium alginate solution in endoscopic submucosal dissection for esophageal and gastric neoplastic lesion: A randomized controlled study
Abstract
Objectives: Sodium alginate (SA) solution has characteristic viscoelasticity. We aimed to determine efficacy and safety of 0.6% SA for submucosal injection during endoscopic submucosal dissection (ESD) in patients with localized neoplastic lesion in the esophageal and gastric mucosa.
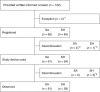
Methods: We conducted a randomized controlled study at six major hospitals in Japan including 130 patients with endoscopically localized neoplastic lesion in the esophageal and gastric mucosa and eligible for ESD. Patients were randomly assigned to SA or 0.4% sodium hyaluronate (SH) group (control); ESD was performed using a submucosal injection of SA/SH. As a primary outcome measure, non-inferiority of SA against SH was investigated using en bloc complete resection in ESD and formation and maintenance of mucosal elevation upon injection as an efficacy index. Adverse events during the study were evaluated as safety outcome measures. This study was registered with Pharmaceuticals and Medical Devices Agency (clinical trial no. 28-277/2016-18; clinical trial identification no. KP2013-009_C001).
Results: Efficacy rate of submucosal injection during ESD was 91.7% (55/60) and 88.7% (55/62) in the SA and SH groups, respectively, demonstrating non-inferiority of SA against SH. Adverse events for which a causal relationship with submucosal injection solution could not be eliminated were noted in 8.2% (5/61) and 4.7% (3/64) in the SA and SH groups, respectively, but symptoms disappeared without treatment/after drug administration in both groups.
Conclusions: In Japan, 0.4% SH is the only commercially approved formulation for submucosal injection during ESD. The study results may expand submucosal injection solution options in clinical practice.
Keywords: endoscopic submucosal dissection; gastric neoplasms; randomized controlled trials; sodium alginate; sodium hyaluronate.
© 2019 The Authors. Digestive Endoscopy published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
The funding for this study was provided by Kaigen Pharma Corporation, Osaka, Japan, which is to receive approval for marketing of the medical device from the Ministry of Health Labor, and Welfare of Japan. The funding source had no role in the design, practice or analysis of this study. Kaigen Pharma Corporation contracted and paid all hospitals on the basis of good clinical practice.
Figures
Comment in
-
Novel submucosal injection solution 0.6% sodium alginate: Bench to market for endoscopic submucosal dissection optimization.Dig Endosc. 2019 Jul;31(4):393-395. doi: 10.1111/den.13426. Epub 2019 Jun 6. Dig Endosc. 2019. PMID: 31034664 No abstract available.
-
Experimental and translational research in gastrointestinal endoscopy, the Japan Gastroenterological Endoscopy Society and perspective.Dig Endosc. 2022 May;34 Suppl 2:129-131. doi: 10.1111/den.14129. Epub 2021 Sep 24. Dig Endosc. 2022. PMID: 34558127 No abstract available.
References
-
- Japanese Gastric Cancer Association . Japanese Gastric Cancer Treatment Guidelines. Tokyo: Kanehara & Co., Ltd, 2014. [Cited 6 Aug 2018.] Available from URL: https://link.springer.com/article/10.1007%2Fs10120-016-0622-4.
-
- The Japan Esophageal Society . Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus. Tokyo: Kanehara & Co., Ltd, 2012. [Cited 14 Aug 2018.] Available from URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4297610/.
-
- Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin. Gastroenterol. Hepatol. 2005; 3: S71–3. - PubMed
-
- Oka S, Tanaka S, Kaneko I et al Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest. Endosc. 2006; 64: 877–83. - PubMed
-
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin. J. Dig. Dis. 2005; 6: 119–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

