Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial
- PMID: 30668322
- PMCID: PMC6990969
- DOI: 10.1016/j.phymed.2018.12.002
Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial
Retraction in
-
Retraction notice to <"Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial"> <[PHYTOMEDICINE 57C (2019) 49-56]>Phytomedicine. 2020 Nov;78:153304. doi: 10.1016/j.phymed.2020.153304. Epub 2020 Sep 21. Phytomedicine. 2020. PMID: 32972797 Free PMC article. No abstract available.
Abstract
Background and objective: Prior small-scale clinical trials showed that Artemisia annua and Artemisia afra infusions, decoctions, capsules, or tablets were low cost, easy to use, and efficient in curing malaria infections. In a larger-scale trial in Kalima district, Democratic Republic of Congo, we aimed to show A. annua and/or A. afra infusions were superior or at least equivalent to artesunate-amodiaquine (ASAQ) against malaria.
Methods: A double blind, randomized clinical trial with 957 malaria-infected patients had two treatment arms: 472 patients for ASAQ and 471 for Artemisia (248 A. annua, 223 A. afra) remained at end of the trial. ASAQ-treated patients were treated per manufacturer posology, and Artemisia-treated patients received 1 l/d of dry leaf/twig infusions for 7 d; both arms had 28 d follow-up. Parasitemia and gametocytes were measured microscopically with results statistically compared among arms for age and gender.
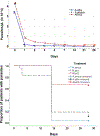
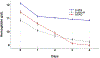
Results: Artemisinin content of A. afra was negligible, but therapeutic responses of patients were similar to A. annua-treated patients; trophozoites cleared after 24 h, but took up to 14 d to clear in ASAQ-treated patients. D28 cure rates defined as absence of parasitemia were for pediatrics 82, 91, and 50% for A. afra, A. annua and ASAQ; while for adults cure rates were 91, 100, and 30%, respectively. Fever clearance took 48 h for ASAQ, but 24 h for Artemisia. From D14-28 no Artemisia-treated patients had microscopically detectable gametocytes, while 10 ASAQ-treated patients remained gametocyte carriers at D28. More females than males were gametocyte carriers in the ASAQ arm but were unaffected in the Artemisia arms. Hemoglobin remained constant at 11 g/dl for A. afra after D1, while for A. annua and ASAQ it decreased to 9-9.5 g/dl. Only 5.0% of Artemisia-treated patients reported adverse effects, vs. 42.8% for ASAQ.
Conclusion: A. annua and A. afra infusions are polytherapies with better outcomes than ASAQ against malaria. In contrast to ASAQ, both Artemisias appeared to break the cycle of malaria by eliminating gametocytes. This study merits further investigation for possible inclusion of Artemisia tea infusions as an alternative for fighting and eradicating malaria.
Keywords: ACT; ASAQ; Artemisinin; Clinical trial; Malaria; Tea infusion.
Copyright © 2018 Elsevier GmbH. All rights reserved.
Conflict of interest statement
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this study that could have influenced its outcome.
Figures


Comment in
-
Comment on "A. annua and A. afra infusions vs. Artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial" Munyangi et al., 2019.Phytomedicine. 2022 Feb;96:152981. doi: 10.1016/j.phymed.2019.152981. Epub 2019 Jun 25. Phytomedicine. 2022. PMID: 31587958 No abstract available.
References
-
- Blanke CH, Naisabha GB, Balemea MB, Mbaruku GM, Heide L, Müller MS, 2008. Herba Artemisiae annuae tea preparation compared to sulfadoxinepyrimethamine in the treatment of uncomplicated falciparum malaria in adults: a randomized double-blind clinical trial. Trop. Doct 38, 113–116. 10.1258/td.2007.060184. - DOI - PubMed
-
- Babiker HA, Schneider P, 2008. Application of molecular methods for monitoring transmission stages of malaria parasites. Biomed. Mater 3 (3), 034007. - PubMed
-
- Chang HM, But PPH, 1986. Pharmacology and Applications of Chinese Materia Media Volume 1 World Scientific Publishing, Singapore.
-
- Chougouo-Kengne RD, Kouamouo J, Mouyou-Somo R, Penge On’ Okoko A, 2012. Etude comparative de l’efficacité thérapeutique de l’Artesunate seul ou en association avec l’amodiaquine et de la tisane de Artemisia annua cultive à l’ouest du Cameroun. Annales de Pharmacie de l’Université de Kinshasa (RDC) 4 (1), 129–150.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

