Top-down inputs drive neuronal network rewiring and context-enhanced sensory processing in olfaction
- PMID: 30668563
- PMCID: PMC6358160
- DOI: 10.1371/journal.pcbi.1006611
Top-down inputs drive neuronal network rewiring and context-enhanced sensory processing in olfaction
Abstract
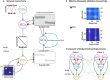
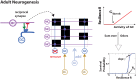
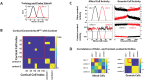
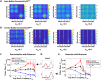
Much of the computational power of the mammalian brain arises from its extensive top-down projections. To enable neuron-specific information processing these projections have to be precisely targeted. How such a specific connectivity emerges and what functions it supports is still poorly understood. We addressed these questions in silico in the context of the profound structural plasticity of the olfactory system. At the core of this plasticity are the granule cells of the olfactory bulb, which integrate bottom-up sensory inputs and top-down inputs delivered by vast top-down projections from cortical and other brain areas. We developed a biophysically supported computational model for the rewiring of the top-down projections and the intra-bulbar network via adult neurogenesis. The model captures various previous physiological and behavioral observations and makes specific predictions for the cortico-bulbar network connectivity that is learned by odor exposure and environmental contexts. Specifically, it predicts that-after learning-the granule-cell receptive fields with respect to sensory and with respect to cortical inputs are highly correlated. This enables cortical cells that respond to a learned odor to enact disynaptic inhibitory control specifically of bulbar principal cells that respond to that odor. For this the reciprocal nature of the granule cell synapses with the principal cells is essential. Functionally, the model predicts context-enhanced stimulus discrimination in cluttered environments ('olfactory cocktail parties') and the ability of the system to adapt to its tasks by rapidly switching between different odor-processing modes. These predictions are experimentally testable. At the same time they provide guidance for future experiments aimed at unraveling the cortico-bulbar connectivity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

