Blood-brain barrier pericytes as a target for HIV-1 infection
- PMID: 30668645
- PMCID: PMC6391611
- DOI: 10.1093/brain/awy339
Blood-brain barrier pericytes as a target for HIV-1 infection
Abstract
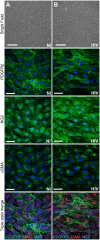
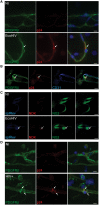
Pericytes are multifunctional cells wrapped around endothelial cells via cytoplasmic processes that extend along the abluminal surface of the endothelium. The interactions between endothelial cells and pericytes of the blood-brain barrier are necessary for proper formation, development, stabilization, and maintenance of the blood-brain barrier. Blood-brain barrier pericytes regulate paracellular flow between cells, transendothelial fluid transport, maintain optimal chemical composition of the surrounding microenvironment, and protect endothelial cells from potential harmful substances. Thus, dysfunction or loss of blood-brain barrier pericytes is an important factor in the pathogenesis of several diseases that are associated with microvascular instability. Importantly, recent research indicates that blood-brain barrier pericytes can be a target of HIV-1 infection able to support productive HIV-1 replication. In addition, blood-brain barrier pericytes are prone to establish a latent infection, which can be reactivated by a mixture of histone deacetylase inhibitors in combination with TNF. HIV-1 infection of blood-brain barrier pericytes has been confirmed in a mouse model of HIV-1 infection and in human post-mortem samples of HIV-1-infected brains. Overall, recent evidence indicates that blood-brain barrier pericytes can be a previously unrecognized HIV-1 target and reservoir in the brain.
Keywords: HIV; HIV reservoirs; HIV-associated neurocognitive disorder; blood–brain barrier pericytes; neuroinfection.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Armulik A, Genové G, Betsholtz C.. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al.Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–61. - PubMed

