Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/mL: week 48 outcomes from ACTG 5353
- PMID: 30668695
- PMCID: PMC6477973
- DOI: 10.1093/jac/dky564
Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/mL: week 48 outcomes from ACTG 5353
Abstract
Background: The AIDS Clinical Trials Group study A5353 demonstrated the efficacy and safety of dolutegravir and lamivudine for initial treatment of HIV-1 infection at week 24 in individuals with HIV-1 RNA 1000-500 000 copies/mL. Optimal ART for treatment-naive individuals must be durable.
Objectives: The aim of this study was to estimate the efficacy and safety of dolutegravir plus lamivudine at week 48 and compare the efficacy in participants with baseline HIV-1 RNA ≤100 000 copies/mL versus >100 000 copies/mL.
Methods: Virological success was defined as HIV-1 RNA <50 copies/mL by FDA Snapshot criteria. Definition of virological failure included confirmed HIV-1 RNA >200 copies/mL at week 24 or later. The proportion of participants with virological success was estimated using two-sided exact Clopper-Pearson 95% CI. Comparison between screening HIV-1 RNA (≤100 000 versus >100 000 copies/mL) strata was carried out by Fisher's exact test. The study was registered with ClinicalTrials.gov, number NCT02582684.
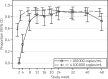
Results: A total of 120 enrolled eligible participants were included in the analysis. At week 48, 102 of the 120 participants (85%; 95% CI 77%-91%) had virological success. Virological success was similar between screening HIV-1 RNA groups. Six (5%) participants had virological non-success and one additional participant experienced virological failure while on study but off study treatment. No new drug resistance mutations were observed. Six (5%) participants had study-related grade 3 or higher adverse events and none discontinued study treatment.
Conclusions: These results add to the evidence that dolutegravir plus lamivudine is a safe and effective option for initial ART in individuals with HIV-1 RNA <500 000 copies/mL.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Figueroa MI, Rolón MJ, Patterson P. et al. Dolutegravir–lamivudine as initial therapy in HIV-infected, ARV naive patients: 96 week results of the PADDLE trial In: Abstracts of Ninth International AIDS Society Conference on HIV Science, Paris, France ,2017. Abstract MOPEB0287. International AIDS Society, Geneva, Switzerland. - PMC - PubMed
-
- Chesney MA, Ickovics JR, Chambers DB. et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 2000; 12: 255–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 AI050409/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069503/AI/NIAID NIH HHS/United States
- UL1 TR001111/TR/NCATS NIH HHS/United States
- U01 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- P30 AI117943/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States