A new class of biological materials: Cell membrane-derived hydrogel scaffolds
- PMID: 30669015
- PMCID: PMC6369705
- DOI: 10.1016/j.biomaterials.2019.01.020
A new class of biological materials: Cell membrane-derived hydrogel scaffolds
Abstract
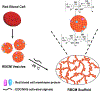
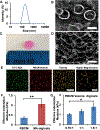
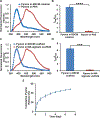
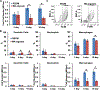
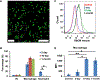
Biological materials are superior to synthetic biomaterials in biocompatibility and active interactions with cells. Here, a new class of biological materials, cell membrane-derived hydrogel scaffolds are reported for harnessing these advantages. To form macroporous scaffolds, vesicles derived from red blood cell membranes (RBCMs) are chemically crosslinked via cryogelation. The RBCM scaffolds with a pore size of around 70 μm are soft and injectable. Highly biocompatible scaffolds are typically made of superhydrophilic polymers and lack the ability to encapsulate and release hydrophobic drugs in a controlled manner. However, hydrophobic molecules can be efficiently encapsulated inside RBCM scaffolds and be sustainedly released. RBCM scaffolds show low neutrophil infiltration after subcutaneous injection in mice, and a significantly higher number of infiltrated macrophages than methacrylate alginate (MA-alginate) scaffolds. According to gene expression and surface markers, these macrophages have an M2-like phenotype, which is anti-inflammatory and immune suppressive. There are also higher percentages of macrophages presenting immunosuppressive PD-L1 in RBCM-scaffolds than in MA-alginate scaffolds. Interestingly, the concentrations of anti-inflammatory cytokine, IL-10 in both types of scaffolds are higher than those in normal organ tissues. This study sheds light on cell membrane-derived hydrogels, which can actively modulate cells in unique ways unavailable to existing hydrogel scaffolds.
Keywords: Drug delivery; Immune modulation; Immunoengineering; Regenerative medicine; Tissue regeneration.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP, Regeneration and orthotopic transplantation of a bioartificial lung, Nat. Med 16(8) (2010) 927–933. - PubMed
-
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA, Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart, Nat. Med 14(2) (2008) 213–221. - PubMed
-
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K, Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix, Nat. Med 16(7) (2010) 814–820. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

