Development and Validation of a LC⁻MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma
- PMID: 30669503
- PMCID: PMC6359656
- DOI: 10.3390/molecules24020360
Development and Validation of a LC⁻MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma
Abstract
Few high-performance liquid chromatography⁻tandem mass spectrometry (LC-MS/MS) methods have been developed for the full quantitation of fatty acids from human plasma without derivatization. Therefore, we propose a method that requires fewer sample preparation steps, which can be used for the quantitation of several polyunsaturated fatty acids in human plasma. The method offers rapid, accurate, sensitive, and simultaneous quantification of omega 3 (α-linolenic, eicosapentaenoic, and docosahexaenoic acids) and omega 6 fatty acids (arachidonic and linoleic acids) using high-performance LC-MS/MS. The selected fatty acids were analysed in lipid extracts from both free and total forms. Chromatographic separation was achieved using a reversed phase C18 column with isocratic flow using ammonium acetate for improving negative electrospray ionization (ESI) response. Mass detection was performed in multiple reaction monitoring (MRM) mode, and deuterated internal standards were used for each target compound. The limits of quantification were situated in the low nanomolar range, excepting linoleic acid, for which the limit was in the high nanomolar range. The method was validated according to the U.S. Department of Health and Human Services guidelines, and offers a fast, sensitive, and reliable quantification of selected omega 3 and 6 fatty acids in human plasma.
Keywords: C18 column; MRM; PUFA; high-performance liquid chromatography; human plasma; metabolomics; negative electrospray ionization; polyunsaturated fatty acids; tandem mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
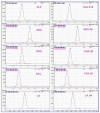
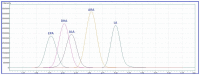
References
-
- Chew E.Y., Clemons T.E., SanGiovanni J.P., Danis R., Ferris F.L., Elman M., Antoszyk A., Ruby A., Orth D., Bressler S., et al. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. J. Am. Med. Assoc. 2013;309:2005–2110. doi: 10.1001/jama.2013.4997. - DOI - PubMed
-
- Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., et al. Associations of Omega-3 Fatty Acid Supplement Use with Cardiovascular Disease Risks Meta-Analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 2018 doi: 10.1001/jamacardio.2017.5205. - DOI - PMC - PubMed
-
- Gonzalez-Periz A., Horrillo R., Ferre N., Gronert K., Dong B., Moran-Salvador E., Titos E., Martinez-Clemente M., Lopez-Parra M., Arroyo V., et al. Obesity-Induced Insulin Resistance and Hepatic Steatosis Are Alleviated by −3 Fatty Acids: A Role for Resolvins and Protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

