Bactericidal and Cytotoxic Properties of Silver Nanoparticles
- PMID: 30669621
- PMCID: PMC6359645
- DOI: 10.3390/ijms20020449
Bactericidal and Cytotoxic Properties of Silver Nanoparticles
Abstract
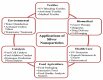
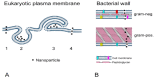
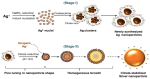
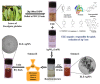
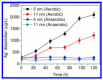
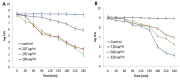
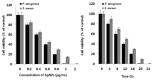
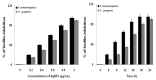
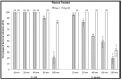
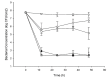
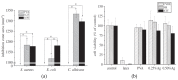
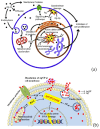
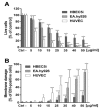
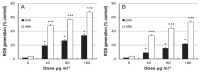
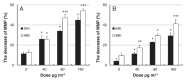
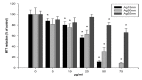
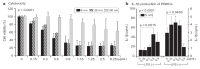
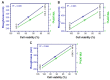
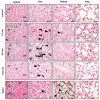
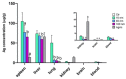
Silver nanoparticles (AgNPs) can be synthesized from a variety of techniques including physical, chemical and biological routes. They have been widely used as nanomaterials for manufacturing cosmetic and healthcare products, antimicrobial textiles, wound dressings, antitumor drug carriers, etc. due to their excellent antimicrobial properties. Accordingly, AgNPs have gained access into our daily life, and the inevitable human exposure to these nanoparticles has raised concerns about their potential hazards to the environment, health, and safety in recent years. From in vitro cell cultivation tests, AgNPs have been reported to be toxic to several human cell lines including human bronchial epithelial cells, human umbilical vein endothelial cells, red blood cells, human peripheral blood mononuclear cells, immortal human keratinocytes, liver cells, etc. AgNPs induce a dose-, size- and time-dependent cytotoxicity, particularly for those with sizes ≤10 nm. Furthermore, AgNPs can cross the brain blood barrier of mice through the circulation system on the basis of in vivo animal tests. AgNPs tend to accumulate in mice organs such as liver, spleen, kidney and brain following intravenous, intraperitoneal, and intratracheal routes of administration. In this respect, AgNPs are considered a double-edged sword that can eliminate microorganisms but induce cytotoxicity in mammalian cells. This article provides a state-of-the-art review on the synthesis of AgNPs, and their applications in antimicrobial textile fabrics, food packaging films, and wound dressings. Particular attention is paid to the bactericidal activity and cytotoxic effect in mammalian cells.
Keywords: administration route; bacteria; cell culture; cytotoxicity; food packaging; membrane; polymer nanocomposite; reactive oxygen species; silver ion; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Tjong S.C., Chen H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004;45:1–88. doi: 10.1016/j.mser.2004.07.001. - DOI
-
- He L.X., Tjong S.C. Aqueous graphene oxide-dispersed carbon nanotubes as inks for the scalable production of all-carbon transparent conductive films. J. Mater. Chem. C. 2016;4:7043–7051. doi: 10.1039/C6TC01224H. - DOI
-
- He L.X., Tjong S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016;109:1–101. doi: 10.1016/j.mser.2016.08.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

