Risk of transfusion-transmitted malaria: evaluation of commercial ELISA kits for the detection of anti-Plasmodium antibodies in candidate blood donors
- PMID: 30670018
- PMCID: PMC6341736
- DOI: 10.1186/s12936-019-2650-0
Risk of transfusion-transmitted malaria: evaluation of commercial ELISA kits for the detection of anti-Plasmodium antibodies in candidate blood donors
Abstract
Background: Transfusion with Plasmodium-infected blood represents a risk for malaria transmission, a rare but severe event. Several non-endemic countries implement a strategy for the screening of candidate blood donors including questionnaire for the identification of at-risk subjects and laboratory testing of blood samples, often serology-based, with temporary deferral from donation for individuals with a positive result. In Italy, the most recent legislation, issued in November 2015, introduced the use of serological tests for the detection of anti-Plasmodium antibodies.
Methods: In the absence of a gold standard for malaria serology, the aim of this work was to evaluate five commercial ELISA kits, and to determine their accuracy (sensitivity and specificity) in comparison to immuno-fluorescence antibody test (IFAT), and their agreement (concordance of results). Serum samples from malaria patients or from subjects with malaria history (N = 64), malaria naïve patients with other parasitic infections (N = 15), malaria naïve blood donors (N = 8) and malaria exposed candidate blood donors (N = 36) were tested.
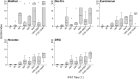
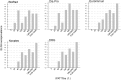
Results: The specificity of all ELISA kits was 100%, while sensitivity ranged between 53 and 64% when compared to IFAT on malaria patients samples. When tested on candidate blood donors' samples, ELISA kits showed highly variable agreement (42-94%) raising the possibility that the same individual could be included or excluded from donation depending on the test in use by the transfusion centre.
Conclusions: These preliminary results indicate how the lack of a gold standard for malaria serology must be taken into account in the application and future revision of current legislation. There is need of developing more sensitive serological assays. Moreover, the adoption of a unique serological test at national level is recommended, as well as the development of screening algorithms based on multiple laboratory tests, including molecular assays.
Keywords: ELISA; IFAT; Plasmodium; Transfusion transmitted malaria.
Figures

Similar articles
-
Multicentric evaluation of the DiaMed enzyme-linked immunosorbent assay malaria antibody test for screening of blood donors for malaria.Vox Sang. 2008 Jan;94(1):33-40. doi: 10.1111/j.1423-0410.2007.00998.x. Epub 2007 Nov 16. Vox Sang. 2008. PMID: 18021184
-
A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies.Malar J. 2007 Feb 21;6:19. doi: 10.1186/1475-2875-6-19. Malar J. 2007. PMID: 17313669 Free PMC article.
-
Comparative evaluation of a rapid diagnostic test, an antibody ELISA, and a pLDH ELISA in detecting asymptomatic malaria parasitaemia in blood donors in Buea, Cameroon.Infect Dis Poverty. 2017 Aug 1;6(1):103. doi: 10.1186/s40249-017-0314-2. Infect Dis Poverty. 2017. PMID: 28760158 Free PMC article.
-
The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria.Transfus Med Rev. 2005 Jul;19(3):229-40. doi: 10.1016/j.tmrv.2005.02.004. Transfus Med Rev. 2005. PMID: 16010653 Review.
-
[Transfusion-transmitted malaria, preventive measures].Transfus Clin Biol. 2005 Jun;12(2):107-13. doi: 10.1016/j.tracli.2005.04.014. Transfus Clin Biol. 2005. PMID: 15907390 Review. French.
Cited by
-
Transfusion-Transmitted Malaria of Plasmodium malariae in Palermo, Sicily.Healthcare (Basel). 2021 Nov 16;9(11):1558. doi: 10.3390/healthcare9111558. Healthcare (Basel). 2021. PMID: 34828604 Free PMC article.
-
Screening blood donors for malaria, can we increase the number of eligible donors? An observational retrospective study.Malar J. 2024 Jun 6;23(1):179. doi: 10.1186/s12936-024-04966-3. Malar J. 2024. PMID: 38844954 Free PMC article.
-
Redefining serological diagnostics with immunoaffinity proteomics.Clin Proteomics. 2023 Oct 12;20(1):42. doi: 10.1186/s12014-023-09431-y. Clin Proteomics. 2023. PMID: 37821808 Free PMC article. Review.
-
Transfusion-Transmitted Malaria and Mitigation Strategies in Nonendemic Regions.Transfus Med Hemother. 2022 Jul 15;49(4):205-217. doi: 10.1159/000525414. eCollection 2022 Aug. Transfus Med Hemother. 2022. PMID: 36159954 Free PMC article. Review.
-
Dynamics of anti-malarial antibodies in non-immune patients during and after a first and unique Plasmodium falciparum malaria episode.Malar J. 2020 Jun 26;19(1):228. doi: 10.1186/s12936-020-03300-x. Malar J. 2020. PMID: 32590983 Free PMC article.
References
-
- WHO . World malaria report 2017. Geneva: World Health Organization; 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

