Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma
- PMID: 30670050
- PMCID: PMC6341618
- DOI: 10.1186/s13024-018-0303-3
Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma
Abstract
Background: Glaucoma is characterized by the progressive dysfunction and loss of retinal ganglion cells. Recent work in animal models suggests that a critical neuroinflammatory event damages retinal ganglion cell axons in the optic nerve head during ocular hypertensive injury. We previously demonstrated that monocyte-like cells enter the optic nerve head in an ocular hypertensive mouse model of glaucoma (DBA/2 J), but their roles, if any, in mediating axon damage remain unclear.
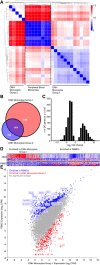
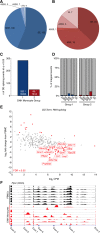
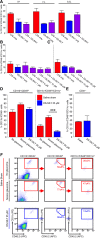
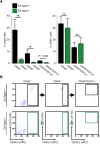
Methods: To understand the function of these infiltrating monocyte-like cells, we used RNA-sequencing to profile their transcriptomes. Based on their pro-inflammatory molecular signatures, we hypothesized and confirmed that monocyte-platelet interactions occur in glaucomatous tissue. Furthermore, to test monocyte function we used two approaches to inhibit their entry into the optic nerve head: (1) treatment with DS-SILY, a peptidoglycan that acts as a barrier to platelet adhesion to the vessel wall and to monocytes, and (2) genetic targeting of Itgam (CD11b, an immune cell receptor that enables immune cell extravasation).
Results: Monocyte specific RNA-sequencing identified novel neuroinflammatory pathways early in glaucoma pathogenesis. Targeting these processes pharmacologically (DS-SILY) or genetically (Itgam / CD11b knockout) reduced monocyte entry and provided neuroprotection in DBA/2 J eyes.
Conclusions: These data demonstrate a key role of monocyte-like cell extravasation in glaucoma and demonstrate that modulating neuroinflammatory processes can significantly lessen optic nerve injury.
Keywords: Extravasation; Glaucoma; Monocyte; Neuroinflammation; Optic nerve; Platelet; RNA-sequencing; Retinal ganglion cell; Vascular leakage.
Conflict of interest statement
Ethics approval
All breeding and experimental procedures were undertaken in accordance with the Association for Research for Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Research. The Institutional Biosafety Committee (IBC) and the Animal Care and Use Committee (ACUC) at The Jackson Laboratory approved this study.
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR, John SWM. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A. 2017;114(19):E3839–E3848. doi: 10.1073/pnas.1608769114. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

