Efficacy of Human-Simulated Epithelial Lining Fluid Exposure of Meropenem-Nacubactam Combination against Class A Serine β-Lactamase-Producing Enterobacteriaceae in the Neutropenic Murine Lung Infection Model
- PMID: 30670411
- PMCID: PMC6437545
- DOI: 10.1128/AAC.02382-18
Efficacy of Human-Simulated Epithelial Lining Fluid Exposure of Meropenem-Nacubactam Combination against Class A Serine β-Lactamase-Producing Enterobacteriaceae in the Neutropenic Murine Lung Infection Model
Abstract
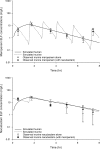
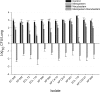
Nacubactam is a novel, broad-spectrum, β-lactamase inhibitor that is currently under development as combination therapy with meropenem. This study evaluated the efficacy of human-simulated epithelial lining fluid (ELF) exposures of meropenem, nacubactam, and the combination of meropenem and nacubactam against class A serine carbapenemase-producing Enterobacteriaceae isolates in the neutropenic murine lung infection model. Twelve clinical meropenem-resistant Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae isolates, all harboring KPC or IMI-type β-lactamases, were utilized in the study. Meropenem, nacubactam, and meropenem-nacubactam (1:1) combination MICs were determined in triplicate via broth microdilution. At 2 h after intranasal inoculation, neutropenic mice were dosed with regimens that provided ELF profiles mimicking those observed in humans given meropenem at 2 g every 8 h and/or nacubactam at 2 g every 8 h (1.5-h infusions), alone or in combination. Efficacy was assessed as the change in bacterial growth at 24 h, compared with 0-h controls. Meropenem, nacubactam, and meropenem-nacubactam MICs were 8 to >64 μg/ml, 2 to >256 μg/ml, and 0.5 to 4 μg/ml, respectively. The average bacterial density at 0 h across all isolates was 6.31 ± 0.26 log10 CFU/lung. Relative to the 0-h control, the mean values of bacterial growth at 24 h in the untreated control, meropenem human-simulated regimen treatment, and nacubactam human-simulated regimen treatment groups were 2.91 ± 0.27, 2.68 ± 0.42, and 1.73 ± 0.75 log10 CFU/lung, respectively. The meropenem-nacubactam combination human-simulated regimen resulted in reductions of -1.50 ± 0.59 log10 CFU/lung. Meropenem-nacubactam human-simulated ELF exposure produced enhanced efficacy against all class A serine carbapenemase-producing Enterobacteriaceae isolates tested in the neutropenic murine lung infection model.
Keywords: Gram negative; OP0595; RG6080; carbapenemase; lung epithelial lining fluid; nacubactam; β-lactam; β-lactamase inhibitor.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Meropenem-nacubactam activity against AmpC-overproducing and KPC-expressing Pseudomonas aeruginosa in a neutropenic murine lung infection model.Int J Antimicrob Agents. 2020 Feb;55(2):105838. doi: 10.1016/j.ijantimicag.2019.10.019. Epub 2019 Nov 6. Int J Antimicrob Agents. 2020. PMID: 31705960
-
In Vivo Efficacy of Meropenem with a Novel Non-β-Lactam-β-Lactamase Inhibitor, Nacubactam, against Gram-Negative Organisms Exhibiting Various Resistance Mechanisms in a Murine Complicated Urinary Tract Infection Model.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e02596-17. doi: 10.1128/AAC.02596-17. Print 2018 Sep. Antimicrob Agents Chemother. 2018. PMID: 30012751 Free PMC article.
-
Pharmacodynamic evaluation of meropenem, cefepime, or aztreonam combined with a novel β-lactamase inhibitor, nacubactam, against carbapenem-resistant and/or carbapenemase-producing Klebsiella pneumoniae and Escherichia coli using a murine thigh-infection model.Int J Antimicrob Agents. 2021 May;57(5):106330. doi: 10.1016/j.ijantimicag.2021.106330. Epub 2021 Mar 28. Int J Antimicrob Agents. 2021. PMID: 33789129
-
Meropenem-vaborbactam: a carbapenem and beta-lactamase inhibitor with activity against carbapenem-resistant Enterobacteriaceae.Eur J Clin Microbiol Infect Dis. 2018 Aug;37(8):1411-1419. doi: 10.1007/s10096-018-3260-4. Epub 2018 Apr 19. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29675787 Review.
-
Meropenem/vaborbactam: a next generation β-lactam β-lactamase inhibitor combination.Expert Rev Anti Infect Ther. 2020 Jul;18(7):643-655. doi: 10.1080/14787210.2020.1756775. Epub 2020 May 3. Expert Rev Anti Infect Ther. 2020. PMID: 32297801 Review.
Cited by
-
Variability in Zinc Concentration among Mueller-Hinton Broth Brands: Impact on Antimicrobial Susceptibility Testing of Metallo-β-Lactamase-Producing Enterobacteriaceae.J Clin Microbiol. 2020 Nov 18;58(12):e02019-20. doi: 10.1128/JCM.02019-20. Print 2020 Nov 18. J Clin Microbiol. 2020. PMID: 32999009 Free PMC article.
-
Antibiotic Therapy Strategies for Treating Gram-Negative Severe Infections in the Critically Ill: A Narrative Review.Antibiotics (Basel). 2023 Jul 31;12(8):1262. doi: 10.3390/antibiotics12081262. Antibiotics (Basel). 2023. PMID: 37627683 Free PMC article. Review.
-
Simulated achievement rate of β-lactams/nacubactam treatment in humans using instantaneous MIC-based PK/PD analysis.J Antimicrob Chemother. 2025 Feb 3;80(2):547-553. doi: 10.1093/jac/dkae443. J Antimicrob Chemother. 2025. PMID: 39665259 Free PMC article.
-
Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections.Pharmaceutics. 2025 Jun 11;17(6):770. doi: 10.3390/pharmaceutics17060770. Pharmaceutics. 2025. PMID: 40574082 Free PMC article.
-
β-Lactam/β-Lactamase Inhibitor Combination Antibiotics Under Development.Pathogens. 2025 Feb 8;14(2):168. doi: 10.3390/pathogens14020168. Pathogens. 2025. PMID: 40005543 Free PMC article. Review.
References
-
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi:10.1093/cid/ciw353. - DOI - PMC - PubMed
-
- Deshpande LM, Rhomberg PR, Sader HS, Jones RN. 2006. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States medical centers: report from the MYSTIC Program (1999–2005). Diagn Microbiol Infect Dis 56:367–372. doi:10.1016/j.diagmicrobio.2006.07.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

