Pharmacokinetics, Safety, and Dosing of Novel Pediatric Levofloxacin Dispersible Tablets in Children with Multidrug-Resistant Tuberculosis Exposure
- PMID: 30670421
- PMCID: PMC6437514
- DOI: 10.1128/AAC.01865-18
Pharmacokinetics, Safety, and Dosing of Novel Pediatric Levofloxacin Dispersible Tablets in Children with Multidrug-Resistant Tuberculosis Exposure
Abstract
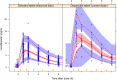
This study characterized the pharmacokinetics of novel 100-mg levofloxacin dispersible tablets in 24 children aged <5 years who had household multidrug-resistant tuberculosus (MDR-TB) exposure. The current data were pooled with previously published data from children (n = 109) with MDR-TB receiving adult (250-mg) levofloxacin tablets, using nonlinear mixed-effects modelling. The adult tablets had 41% lower bioavailability than the novel dispersible tablets, resulting in much higher exposures with the dispersible tablets with the same dose.
Keywords: bioavailability; children; dispersible tablets; formulation; levofloxacin; multidrug-resistant tuberculosis; pharmacokinetics.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- U.S. Food and Drug Administration. 2008. Levofloxacin prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_0206.... Accessed 17 April 2014.
-
- Thee S, Garcia-Prats AJ, McIlleron HM, Wiesner L, Castel S, Norman J, Draper HR, van der Merwe PL, Hesseling AC, Schaaf HS. 2014. Pharmacokinetics of ofloxacin and levofloxacin for prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother 58:2948–2951. doi:10.1128/AAC.02755-13. - DOI - PMC - PubMed
-
- Mase SR, Jereb JA, Gonzalez D, Martin F, Daley CL, Fred D, Loeffler AM, Menon LR, Bamrah Morris S, Brostrom R, Chorba T, Peloquin CA. 2016. Pharmacokinetics and dosing of levofloxacin in children treated for active or latent multidrug-resistant tuberculosis, Federated States of Micronesia and Republic of the Marshall Islands. Pediatr Infect Dis J 35:414–421. doi:10.1097/INF.0000000000001022. - DOI - PMC - PubMed
-
- Denti P, Garcia-Prats AJ, Draper HR, Wiesner L, Winckler J, Thee S, Dooley KE, Savic RM, McIlleron HM, Schaaf HS, Hesseling AC. 2018. Levofloxacin population pharmacokinetics in South African children treated for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 62: e01521-17. doi:10.1128/AAC.01521-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

