The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum
- PMID: 30670466
- PMCID: PMC6719602
- DOI: 10.1101/cshperspect.a033886
The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum
Abstract
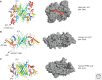
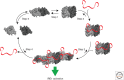
Most of the secreted and plasma membrane proteins are synthesized on membrane-bound ribosomes on the endoplasmic reticulum (ER). They require engagement of ER-resident chaperones and foldases that assist in their folding and maturation. Since protein homeostasis in the ER is crucial for cellular function, the protein-folding status in the organelle's lumen is continually surveyed by a network of signaling pathways, collectively called the unfolded protein response (UPR). Protein-folding imbalances, or "ER stress," are detected by highly conserved sensors that adjust the ER's protein-folding capacity according to the physiological needs of the cell. We review recent developments in the field that have provided new insights into the ER stress-sensing mechanisms used by UPR sensors and the mechanisms by which they integrate various cellular inputs to adjust the folding capacity of the organelle to accommodate to fluctuations in ER protein-folding demands.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Adamson B, Norman TM, Jost M, Cho MY, Nunez JK, Chen Y, Villalta JE, Gilbert LA, Horlbeck MA, Hein MY, et al. 2016. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167: 1867–1882.e21. 10.1016/j.cell.2016.11.048 - DOI - PMC - PubMed
