A new LC-MS assay for the quantitative analysis of vitamin K metabolites in human urine
- PMID: 30670472
- PMCID: PMC6446701
- DOI: 10.1194/jlr.D087916
A new LC-MS assay for the quantitative analysis of vitamin K metabolites in human urine
Abstract
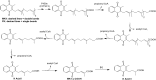
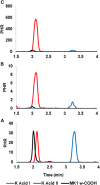
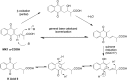
Vitamin K (VK), in both its phylloquinone and menaquinone forms, has been hypothesized to undergo ω- and β-oxidation on its hydrophobic side chain in order to generate the observed urinary metabolites, K acid I and K acid II, which are excreted primarily as glucuronide conjugates. Synthetic standards of K acid I, K acid II, and a putative intermediate metabolite, menaquinone (MK)1 ω-COOH, were used to develop and optimize a new atmospheric pressure negative chemical ionization LC-MS/MS assay for the quantitation of these compounds in urine from untreated individuals and subjects treated with a high dose VK supplement. VK catabolites were extracted from urine, deconjugated, and converted to their methyl ester derivatives using previously reported methodology. The assay showed a high degree of sensitivity, with limits of detection below 10-50 fmol of metabolite per milliliter of urine, as well as an inter-assay precision of 8-12%. Metabolite standards provided unambiguous evidence for MK1 ω-COOH as a new human urinary metabolite of VK. This assay provides a minimally invasive, highly sensitive, and specific alternative for monitoring VK status in humans.
Keywords: beta-oxidation; liquid chromatography-mass spectrometry; menaquinone; omega-oxidation; phylloquinone.
Copyright © 2019 McDonald et al.
Figures

References
-
- Booth S. L., and Suttie J. W.. 1998. Dietary intake and adequacy of vitamin K. J. Nutr. 128: 785–788. - PubMed
-
- Nakagawa K., Hirota Y., Sawada N., Yuge N., Watanabe M., Uchino Y., Okuda N., Shimomura Y., Suhara Y., and Okano T.. 2010. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 468: 117–121. - PubMed
-
- Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., and Nakagawa K.. 2008. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283: 11270–11279. - PubMed
-
- Thijssen H. H., Vervoort L. M., Schurgers L. J., and Shearer M. J.. 2006. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 95: 260–266. - PubMed
-
- Elder S. J., Haytowitz D. B., Howe J., Peterson J. W., and Booth S. L.. 2006. Vitamin K contents of meat, dairy, and fast food in the U.S. diet. J. Agric. Food Chem. 54: 463–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

