Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 Is Essential for Proinsulin Handling
- PMID: 30670477
- PMCID: PMC6425875
- DOI: 10.2337/db18-0671
Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 Is Essential for Proinsulin Handling
Abstract
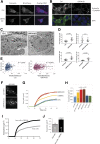
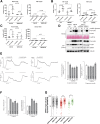
Although endoplasmic reticulum (ER) chaperone binding to mutant proinsulin has been reported, the role of protein chaperones in the handling of wild-type proinsulin is underinvestigated. Here, we have explored the importance of glucose-regulated protein 94 (GRP94), a prominent ER chaperone known to fold insulin-like growth factors, in proinsulin handling within β-cells. We found that GRP94 coimmunoprecipitated with proinsulin and that inhibition of GRP94 function and/or expression reduced glucose-dependent insulin secretion, shortened proinsulin half-life, and lowered intracellular proinsulin and insulin levels. This phenotype was accompanied by post-ER proinsulin misprocessing and higher numbers of enlarged insulin granules that contained amorphic material with reduced immunogold staining for mature insulin. Insulin granule exocytosis was accelerated twofold, but the secreted insulin had diminished bioactivity. Moreover, GRP94 knockdown or knockout in β-cells selectively activated protein kinase R-like endoplasmic reticulum kinase (PERK), without increasing apoptosis levels. Finally, GRP94 mRNA was overexpressed in islets from patients with type 2 diabetes. We conclude that GRP94 is a chaperone crucial for proinsulin handling and insulin secretion.
© 2019 by the American Diabetes Association.
Figures




References
-
- Rubenstein AH, Melani F, Pilkis S, Steiner DF. . Proinsulin. Secretion, metabolism, immunological and biological properties. Postgrad Med J 1969;45 (Suppl.):476–481 - PubMed
-
- Winter J, Klappa P, Freedman RB, Lilie H, Rudolph R. Catalytic activity and chaperone function of human protein-disulfide isomerase are required for the efficient refolding of proinsulin. J Biol Chem 2002;277:310–317 - PubMed
-
- Goodge KA, Hutton JC. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Semin Cell Dev Biol 2000;11:235–242 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

