Engineering Strategies to Boost Crop Productivity by Cutting Respiratory Carbon Loss
- PMID: 30670486
- PMCID: PMC6447004
- DOI: 10.1105/tpc.18.00743
Engineering Strategies to Boost Crop Productivity by Cutting Respiratory Carbon Loss
Abstract
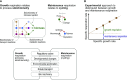
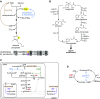
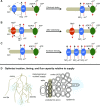
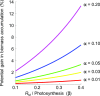
Roughly half the carbon that crop plants fix by photosynthesis is subsequently lost by respiration. Nonessential respiratory activity leading to unnecessary CO2 release is unlikely to have been minimized by natural selection or crop breeding, and cutting this large loss could complement and reinforce the currently dominant yield-enhancement strategy of increasing carbon fixation. Until now, however, respiratory carbon losses have generally been overlooked by metabolic engineers and synthetic biologists because specific target genes have been elusive. We argue that recent advances are at last pinpointing individual enzyme and transporter genes that can be engineered to (1) slow unnecessary protein turnover, (2) replace, relocate, or reschedule metabolic activities, (3) suppress futile cycles, and (4) make ion transport more efficient, all of which can reduce respiratory costs. We identify a set of engineering strategies to reduce respiratory carbon loss that are now feasible and model how implementing these strategies singly or in tandem could lead to substantial gains in crop productivity.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures

References
-
- Ainsworth E.A., Long S.P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165: 351–371. - PubMed
-
- Alonso A.P., Vigeolas H., Raymond P., Rolin D., Dieuaide-Noubhani M. (2005). A new substrate cycle in plants. Evidence for a high glucose-phosphate-to-glucose turnover from in vivo steady-state and pulse-labeling experiments with [13C]glucose and [14C]glucose. Plant Physiol. 138: 2220–2232. - PMC - PubMed
-
- Amthor J.S. (1989). Respiration and Crop Productivity. (New York: Springer-Verlag; ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

