Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials
- PMID: 30670513
- PMCID: PMC6347863
- DOI: 10.1136/bmjopen-2018-023600
Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials
Abstract
Objective: To assess the benefits and harms of pregabalin in the management of neuropathic pain.
Design: Rapid review and meta-analysis of phase III, randomised, placebo-controlled trials.
Participants: Adults aged 18 years and above with neuropathic pain defined according to the International Association for the Study of Pain criteria.
Interventions: Pregabalin or placebo.
Primary and secondary outcome measures: Our primary outcomes were pain (as measured using validated scales) and adverse events. Our secondary outcomes were sleep disturbance, quality of life, Patient Global Impression of Change, Clinician Global Impression scale, anxiety and depression scores, overall discontinuations and discontinuations because of adverse events.
Results: We included 28 trials comprising 6087 participants. The neuropathic pain conditions studied were diabetic peripheral neuropathy, postherpetic neuralgia, herpes zoster, sciatica (radicular pain), poststroke pain and spinal cord injury-related pain. Patients who took pregabalin reported significant reductions in pain (numerical rating scale (NRS)) compared with placebo (standardised mean difference (SMD) -0.49 (95% CI -0.66 to -0.32, p<0.00001), very low quality evidence). Pregabalin significantly reduced sleep interference scores (NRS) compared with placebo (SMD -0.38 (95% CI -0.50 to -0.26, p<0.00001), moderate quality evidence. Pregabalin significantly increased the risk of adverse events compared with placebo (RR 1.33 (95% CI 1.23 to 1.44, p<0.00001, low quality evidence)). The risks of experiencing weight gain, somnolence, dizziness, peripheral oedema, fatigue, visual disturbances, ataxia, non-peripheral oedema, vertigo and euphoria were significantly increased with pregabalin. Pregabalin was significantly more likely than placebo to lead to discontinuation of the drug because of adverse events (RR 1.91 (95% CI 1.54 to 2.37, p<0.00001), low quality evidence).
Conclusion: Pregabalin has beneficial effects on some symptoms of neuropathic pain. However, its use significantly increases the risk of a number of adverse events and discontinuation due to adverse events. The quality of the evidence from journal publications is low.
Keywords: Pregabalin; benefits; harms; meta-analysis; systematic review.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CJH has received expenses and fees for his media work. He has received expenses from the WHO and FDA and holds grant funding from the NIHR, the NIHR School of Primary Care Research, The Wellcome Trust and the WHO. He has received financial remuneration from an asbestos case. He has also received income from the publication of a series of toolkit books published by Blackwell’s. On occasion, he receives expenses for teaching EBM and is also paid for his general practitioner work in National Health Service out of hours. CEBM jointly runs the EvidenceLive Conference with the BMJ and the Overdiagnosis Conference with some international partners that are based on a non-profit making model. BG receives funding from the Laura and John Arnold Foundation and reports personal fees from intermittent additional personal income from speaking and writing for lay audiences on problems in science and medicine including regulatory shortcomings.
Figures

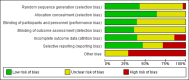
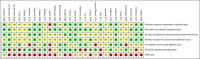


References
-
- OpenPrescribing.net. High-level prescribing trends for Pregabalin (BNF code 0408010AE) across all GP practices in NHS England. 2010. https://openprescribing.net/chemical/0408010AE/ (accessed 8th Mar 2018).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
